추천 제품
분석
98%
refractive index
n20/D 1.347 (lit.)
bp
113 °C/747 mmHg (lit.)
density
1.127 g/mL at 25 °C (lit.)
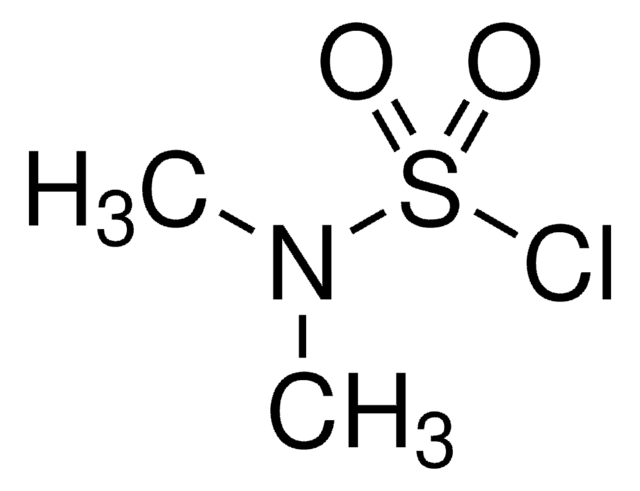
SMILES string
CCCC(=O)OCC(F)(F)F
InChI
1S/C6H9F3O2/c1-2-3-5(10)11-4-6(7,8)9/h2-4H2,1H3
InChI key
DEXWRCYOMLUJRF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Solvent effects on porcine pancreatic lipase-catalyzed transesterification of 2,2,2-trifluoroethyl butyrate has been studied. Kinetics of the reaction of OH radicals and Cl atoms with 2,2,2 trifluoroethyl butyrate has been reported.
애플리케이션
2,2,2-Trifluoroethyl butyrate may be used in the lipase catalyzed resolution of unsubstituted and N-alkyl substituted 2-amino-1-phenylethanols. It may be used in the synthesis of enantiomerically pure (R)- or (S)-1-aminoindane.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
69.8 °F - closed cup
Flash Point (°C)
21 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Tropospheric degradation of 2, 2, 2 trifluoroethyl butyrate: Kinetic study of their reactions with OH radicals and Cl atoms at 298K.
Blanco MB, et al.
Chemical Physics Letters, 578, 33-37 (2013)
Lipase catalysis in the optical resolution of 2-amino-1-phenylethanol derivatives.
Kanerva LT, et al.
Journal of the Chemical Society. Perkin Transactions 1, 14, 1759-1762 (1992)
W H Boesten et al.
Organic letters, 3(8), 1121-1124 (2001-05-12)
[reaction: see text]. Diastereoselective Strecker reactions based on (R)-phenylglycine amide as chiral auxiliary are reported. The Strecker reaction is accompanied by an in situ crystallization-induced asymmetric transformation, whereby one diastereomer selectively precipitates and can be isolated in 76-93% yield and
L T Kanerva et al.
Acta chemica Scandinavica (Copenhagen, Denmark : 1989), 44(10), 1032-1035 (1990-11-01)
Porcine pancreatic lipase-catalysed transesterifications of 2,2,2-trifluoroethyl butyrate with racemic 2-octanol and 1-phenylethanol have been studied in different organic solvents. Solvent hydrophobicity (log P -1.1 to 3.3) has only a minor effect on the reaction rate. Independently of the solvent used
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.