추천 제품
vapor pressure
0.36 psi ( 20 °C)
양식
liquid
재고 정보
available only in USA
refractive index
n20/D 1.474 (lit.)
density
1.131 g/mL at 25 °C (lit.)
작용기
ester
ketone
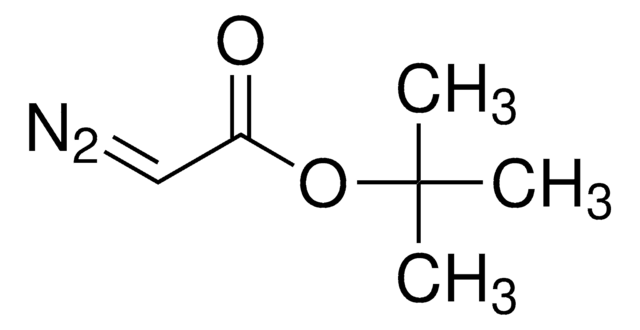
SMILES string
CCOC(=O)C(=[N+]=[N-])C(C)=O
InChI
1S/C6H8N2O3/c1-3-11-6(10)5(8-7)4(2)9/h3H2,1-2H3
InChI key
JWTPSIXYXYNAOU-UHFFFAOYSA-N
일반 설명
애플리케이션
- 1,4-oxathiocines and thiopyran derivatives via Rh-catalyzed reaction with 2-amino-4,5-dihydro-3-thiophenecarbonitriles.[3]
- β-keto esters via C−H insertion reaction with aromatic aldehydes using NbCl5 as a catalyst.[4]
- Diazoacetoacetate derivatives by reacting with aldehydes via aldol condensation and subsequent and in situ oxidation reaction.[5]
- Isoquinolone and pyridone derivatives by Rh-catalyzed C−H activation/annulation reaction with various N-methoxybenzamides.[6]
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Self-react. C - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point (°F)
185.0 °F - closed cup
Flash Point (°C)
85 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.