모든 사진(1)
크기 선택
보기 변경
5 G
₩359,818
About This Item
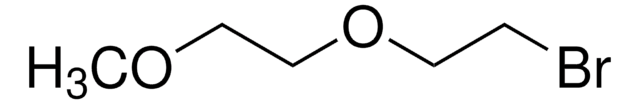
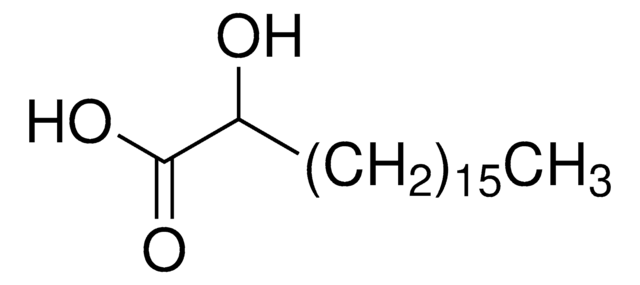
Linear Formula:
HO(CH2)14CO2H
CAS Number:
Molecular Weight:
258.40
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
85-89 °C (lit.)
작용기
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCCCCCC(O)=O
InChI
1S/C15H30O3/c16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15(17)18/h16H,1-14H2,(H,17,18)
InChI key
BZUNJUAMQZRJIP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide.[1] It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.[2]
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives.[3] Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.[4]
애플리케이션
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.[5]
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.[6]
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.[7]
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Sacha Ferdinandusse et al.
Journal of lipid research, 45(6), 1104-1111 (2004-04-03)
Dicarboxylic acids (DCAs) are omega-oxidation products of monocarboxylic acids. After activation by a dicarboxylyl-CoA synthetase, the dicarboxylyl-CoA esters are shortened via beta-oxidation. Although it has been studied extensively where this beta-oxidation process takes place, the intracellular site of DCA oxidation
Lipase catalyzed synthesis of pentadecanolide from 15-hydroxypentadecanoic acid.
Pan XB, et al.
Chinese Journal of Applied Chemistry / Ying Yong Hua Xue, 21(8), 850-852 (2004)
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Dušan Veličković et al.
The Plant journal : for cell and molecular biology, 80(5), 926-935 (2014-10-04)
The cutin polymers of different fruit cuticles (tomato, apple, nectarine) were examined using matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) after in situ release of the lipid monomers by alkaline hydrolysis. The mass spectra were acquired from each coordinate
Zeolite-catalyzed macrolactonization of Ookoshi T and Onaka M. ω-hydroxyalkanoic acids in a highly concentrated solution.
Ookoshi T and Onaka M.
Tetrahedron Letters, 39(3), 293-296 (1998)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)