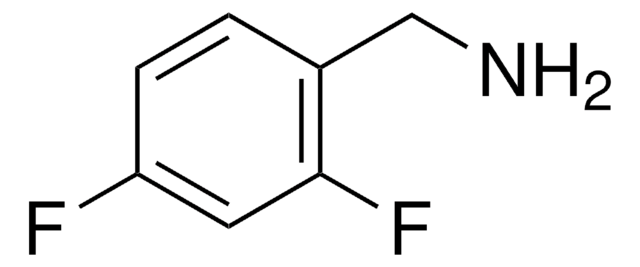
380067
4,4-Dimethoxy-2-butanone
technical grade, ≥90%
동의어(들):
3-Oxobutyraldehyde dimethylacetal, 3-Ketobutyraldehyde dimethyl acetal, 4,4-Dimethoxy-2-butanone, Acetylacetaldehyde dimethylacetal
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
CH3COCH2CH(OCH3)2
CAS Number:
Molecular Weight:
132.16
Beilstein:
1702372
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
technical grade
Quality Level
분석
≥90%
양식
liquid
refractive index
n20/D 1.414 (lit.)
bp
70-73 °C/20 mmHg (lit.)
density
0.996 g/mL at 25 °C (lit.)
작용기
acetal
ether
ketone
SMILES string
COC(CC(C)=O)OC
InChI
1S/C6H12O3/c1-5(7)4-6(8-2)9-3/h6H,4H2,1-3H3
InChI key
PJCCSZUMZMCWSX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4,4-Dimethoxy-2-butanone (Acetylacetaldehyde dimethylacetal) is a ketone.
애플리케이션
4,4-Dimethoxy-2-butanone (β-ketobutyracetal) may be used in the preparation of:
- (R)-4,4-dimethoxy-2-butanol
- pyrazoles and pyrimidines
- [7,16-dihydro- 6,15( 17)-dimetbyldibenzo[b,i]-[1,4,8,11]tetrilazacyclotetradecinato-N5,N9,N14,N18]nickel{II)
4,4-Dimethoxy-2-butanone (Acetylacetaldehyde dimethylacetal) may be used in the preparation of N,N-diethyl-[2-(4-fluorophenyl)-5-methylpyrazolo[1,5-a]-pyrimidin-3-yl]acetamide and acetoacetaldehyde.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
134.6 °F - closed cup
Flash Point (°C)
57 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Olga B Gutiérrez Acosta et al.
Applied and environmental microbiology, 79(20), 6228-6235 (2013-08-06)
Acetone is activated by aerobic and nitrate-reducing bacteria via an ATP-dependent carboxylation reaction to form acetoacetate as the first reaction product. In the activation of acetone by sulfate-reducing bacteria, acetoacetate has not been found to be an intermediate. Here, we
β-keto acetals. I. Synthesis of pyrazoles and pyrimidines and the steric inhibition of resonance in 5-alkyl-1-p-nitrophenylpyrazoles.
Burness DM.
The Journal of Organic Chemistry, 21(1), 97-101 (1956)
Yamadazyma Farinosa IFO 10896-mediated reduction of 4, 4-dimethoxy-2-butanone as the key-step for the preparation of 1, 3-diols with unsymmetrical substituents.
Yamazaki T, et al.
Synthetic Communications, 30(16), 3061-3072 (2000)
2-Arylpyrazolo [1,5-a] pyrimidin-3-yl acetamides. New potent and selective peripheral benzodiazepine receptor ligands.
Selleri S, et al.
BioTechnology: An Indian Journal, 9(10), 2661-2671 (2001)
Facile template synthesis of nickel (II) complexes of dibenzotetraaza [14] annulenes.
Cutler AR, et al.
Inorganic Chemistry, 24(14), 2276-2281 (1985)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.