모든 사진(1)
About This Item
Linear Formula:
C6H5CH2CH2COCOOCH2CH3
CAS Number:
Molecular Weight:
206.24
Beilstein:
2725083
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
375322 제품과 관련한 질문은 현지 머크 영업소 또는 판매 사원에게 문의해 주십시오. 고객지원팀으로 연락바랍니다.
추천 제품
분석
97%
양식
liquid
refractive index
n20/D 1.504 (lit.)
bp
132 °C/2 mmHg (lit.)
density
1.091 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(=O)CCc1ccccc1
InChI
1S/C12H14O3/c1-2-15-12(14)11(13)9-8-10-6-4-3-5-7-10/h3-7H,2,8-9H2,1H3
InChI key
STPXIOGYOLJXMZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
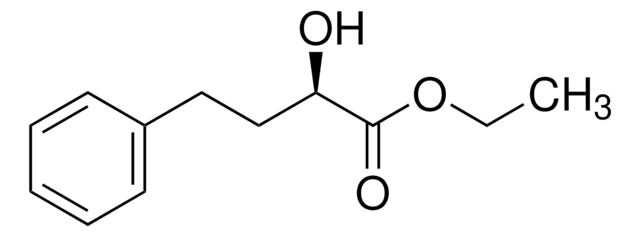
Ethyl 2-oxo-4-phenylbutyrate is an aliphatic α-ketoester.[1] Bioreduction of ethyl 2-oxo-4-phenylbutyrate is reported to yield ethyl (R)-2-hydroxy-4-phenylbutanoate.[2] The effect of ionic liquid on the asymmetric reduction of ethyl 2-oxo-4-phenylbutyrate by Saccharomyces cerevisiae has been reported.[3] Asymmetric reduction of ethyl 2-oxo-4-phenylbutyrate using a bacterial reductase is reported.[4] Enantioselective hydrogenation of ethyl 2-oxo-4-phenylbutyrate using homogeneous Rh-diphosphine and heterogeneous Pt/Al2O3-cinchona catalysts has been reported.
애플리케이션
Ethyl 2-oxo-4-phenylbutyrate may be used in the synthesis of ethyl (R)-2-hydroxy-4-phenylbutyrate, an important chiral precursor for angiotensin-converting enzyme (ACE) inhibitor.[5]
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Gloves
Efficient Reduction of Ethyl 2-Oxo-4-phenylbutyrate at 620 g? L- 1 by a Bacterial Reductase with Broad Substrate Spectrum.
Ni Y, et al.
Advanced Synthesis & Catalysis, 353(8), 1213-1217 (2011)
Nai-Dong Shen et al.
Organic letters, 14(8), 1982-1985 (2012-04-07)
A new reductase, CgKR2, with the ability to reduce ethyl 2-oxo-4-phenylbutyrate (OPBE) to ethyl (R)-2-hydroxy-4-phenylbutyrate ((R)-HPBE), an important chiral precursor for angiotensin-converting enzyme (ACE) inhibitors, was discovered. For the first time, (R)-HPBE with >99% ee was produced via bioreduction of
Ye Ni et al.
Journal of biotechnology, 168(4), 493-498 (2013-10-15)
Ethyl (R)-2-hydroxy-4-phenylbutanoate [(R)-HPBE] is a versatile and important chiral intermediate for the synthesis of angiotensin-converting enzyme (ACE) inhibitors. Recombinant E. coli strain coexpressing a novel NADPH-dependent carbonyl reductase gene iolS and glucose dehydrogenase gene gdh from Bacillus subtilis showed excellent
Yu-Gang Shi et al.
Journal of industrial microbiology & biotechnology, 35(11), 1419-1424 (2008-08-22)
The effect of ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]) on the asymmetric reduction of ethyl 2-oxo-4-phenylbutyrate (EOPB) to synthesize optical active ethyl 2-hydroxy-4-phenylbutyrate (EHPB) catalyzed by Saccharomyces cerevisiae was investigated. (R)-EHPB [70.4%, e.e.(R)] is obtained using ethyl ether or benzene as
Efficient Reduction of Ethyl 2-Oxo-4-phenylbutyrate at 620 g? L- 1 by a Bacterial Reductase with Broad Substrate Spectrum.
Ni Y, et al.
J. Mol. Catal. A: Chem., 107(1), 85-94 (1996)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.