모든 사진(2)
크기 선택
보기 변경
1 G
₩337,082
About This Item
실험식(Hill 표기법):
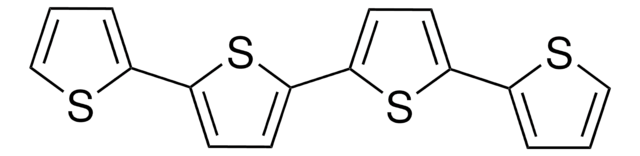
C12H8S3
CAS Number:
Molecular Weight:
248.39
Beilstein:
178604
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
분석
99%
mp
93-95 °C (lit.)
SMILES string
c1csc(c1)-c2ccc(s2)-c3cccs3
InChI
1S/C12H8S3/c1-3-9(13-7-1)11-5-6-12(15-11)10-4-2-8-14-10/h1-8H
InChI key
KXSFECAJUBPPFE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2,2′:5′,2′′-Terthiophene (TTh) may be prepared by nickel catalysed coupling reaction of grignard′s reagent derived from 2-bromothiophene and magnesium.[1] It generates singlet oxygen.[2] In nature, it is found in the floral extract of Tagetes minuta[3] and Echinops grijisii.[4] It is known to be toxic to mosquitoes.[3] It also exihibits antifungal activity.[4]
애플리케이션
3T can be combined with 3,4-ethylenedioxythiophene (EDOT) in a tetrabutylammonium perchlorate solution for use as an electrochromic copolymer for a wide range of applications like photovoltaics[5] and polymer light emitting diodes (LEDs).[6][7] It can also be used to form metal-organic based thin films with metals like aluminum, silver, and calcium which can potentially be used for optoelectronics based applications.[8]
Electrochemical copolymerization of carbazole and TTh in sodium perchlorate/acetonitrile was reported.[9] Electrochromic copolymer based on TTh and 3, 4-ethylenedioxythiophene has been reported.[10] TTh acts as a monomer precursor for polythiophene[11] and as a dopant for polycarbonate.[12] It may function as a photosensitizer.[13]
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Kosuke Sawabe et al.
Advanced materials (Deerfield Beach, Fla.), 24(46), 6141-6146 (2012-09-11)
Extremely high current densities are realized in single-crystal ambipolar light-emitting transistors using an electron-injection buffer layer and a current-confinement structure via laser etching. Moreover, a linear increase in the luminance was observed at current densities of up to 1 kA
Synthetic Metals, 62, 233-233 (1994)
Roderick Pernites et al.
Biosensors & bioelectronics, 26(5), 2766-2771 (2010-11-18)
A novel chemosensitive ultrathin film with high selectivity was developed for the detection of naproxen, paracetamol, and theophylline using non-covalent electropolymerized molecular imprinted polymers (E-MIP). A series of monofunctional and bifunctional H-bonding terthiophene and carbazole monomers were compared for imprinting
Jia Du et al.
ACS applied materials & interfaces, 8(48), 33025-33033 (2016-12-10)
Two new donor-acceptor small molecules based on benzo[1,2-b:4,5-b']dithiophene (BDT) and benzo[c][1,2,5]thiadiazole (BT) were designed and synthesized. Small molecules 4,4'-[(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(2,2'-bithiophene)-5,5'-diyl]bis(benzo[c][1,2,5]thiadiazole) (BDT-TT-BT) and 4,4'-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis[7-(2,2'-bithiophene-5-yl)benzo[c][1,2,5]thiadiazole] (BDT-BT-TT) are structural isomers with the 2,2-bithiophene unit placed either between the BDT and BT units or at
Process Design and Scale-Up of the Synthesis of 2,2`:5`,2``-Terthienyl
Smeets BJJ, et al.
Organic Process Research & Development, 7(1), 10-16 (2003)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
