284734
Indole-3-carboxylic acid
ReagentPlus®, 99%
동의어(들):
β-Indolylcarboxylic acid, 3-Carboxyindole, 3-Indole formic acid, 3-Indolylcarboxylic acid, Indole-β-carboxylic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
Beilstein:
129435
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
제품 라인
ReagentPlus®
분석
99%
양식
solid
mp
232-234 °C (dec.) (lit.)
solubility
95% ethanol: soluble 5%, clear to slightly hazy, light yellow to yellow
SMILES string
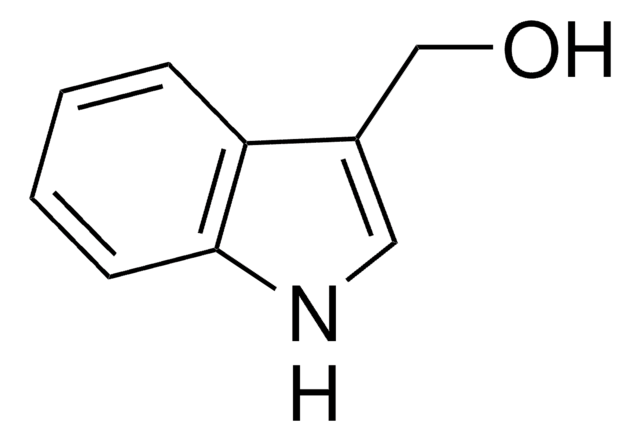
OC(=O)c1c[nH]c2ccccc12
InChI
1S/C9H7NO2/c11-9(12)7-5-10-8-4-2-1-3-6(7)8/h1-5,10H,(H,11,12)
InChI key
KMAKOBLIOCQGJP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The structures of the derivatives of indole-3-carboxylic acid were studied using gas chromatography with high resolution mass spectrometry (GC-HRMS), ultra-high performance liquid chromatography in combination with high resolution tandem mass spectrometry (UHPLC-HRMS), nuclear magnetic resonance spectroscopy (NMR) and Fourier transform infrared spectroscopy (FT-IR).
애플리케이션
Reactant for preparation of:
- Anticancer agents
- Derivatives of amino acids and peptides
- Serotonin 5-HT4 receptor antagonists
- Primary acylureas
- Inhibitors of Gli1-mediated transcription in the Hedgehog pathway
- Serotonin 5-HT6 antagonists
- Very Late Antigen-4 (VLA-4) sntagonists
- EphB3 receptor tyrosine kinase inhibitors
- Potential Therapeutic Agent for Alzheimer′s Disease
- Vinyl ester pseudotripeptide proteasome inhibitors
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Analytical characterization of some synthetic cannabinoids, derivatives of indole-3-carboxylic acid.
Vadim Shevyrin et al.
Forensic science international, 232(1-3), 1-10 (2013-09-24)
By means of gas chromatography with high resolution mass spectrometry (GC-HRMS), ultra-high performance liquid chromatography in combination with high resolution tandem mass spectrometry (UHPLC-HRMS), nuclear magnetic resonance spectroscopy (NMR) and Fourier transform infrared spectroscopy (FT-IR), structure of a series from
M U Ahmad et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 23(9), 841-847 (1985-09-01)
The nitrosation of gramine, a tertiary amine alkaloid present in barley malt, was carried out by reaction with sodium nitrite in buffered acetic acid (pH 3.4) for 1 hr at room temperature. Two major non-volatile products of the nitrosation reaction
Jin-Mo Ku et al.
The Journal of organic chemistry, 72(21), 8115-8118 (2007-09-20)
An enantioselective synthetic method for (-)-cis-clavicipitic acid (1) was reported. 1 was obtained in 10 steps (99% ee and 20% overall yield) from 1H-indole-3-carboxylic acid methyl ester (9) via asymmetric phase-transfer catalytic alkylation and diastereoselective Pd(II)-catalyzed intramolecular aminocyclization as key
Mu-Yang Wang et al.
Journal of integrative plant biology, 54(7), 471-485 (2012-05-26)
Camalexin (3-thiazol-2'-yl-indole) is the major phytoalexin found in Arabidopsis thaliana. Several key intermediates and corresponding enzymes have been identified in camalexin biosynthesis through mutant screening and biochemical experiments. Camalexin is formed when indole-3-acetonitrile (IAN) is catalyzed by the cytochrome P450
Paweł Bednarek
Current opinion in plant biology, 15(4), 407-414 (2012-03-27)
In plants, a host's responses to an attempted infection include activation of various secondary metabolite pathways, some of which are specific for particular plant phylogenetic clades. Phytochemicals that represent respective end products in plant immunity have been stereotypically linked to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.