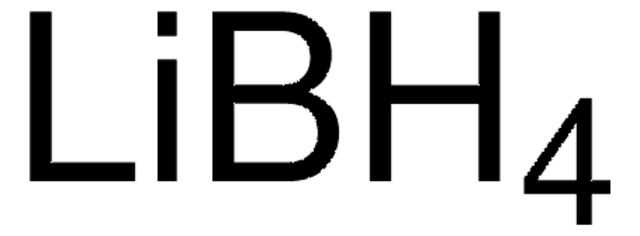추천 제품
형태
liquid
Quality Level
반응 적합성
reagent type: reductant
농도
2.0 M in THF
density
0.896 g/mL at 25 °C
SMILES string
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI key
UUKMSDRCXNLYOO-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Lithium borohydride solution (2M in THF) has been used for the reduction of 5-benzylidene-2,4-thiazolidinediones and 5-benzylidene-4-oxo-2-thiazolidinethiones to form 5-benzyl-2,4-thiazolidinediones and 5-benzyl-4-oxo-2-thiazolidinethiones, respectively.
Reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes
- Mechano-chemical metathesis reactions
- Noncatalytic hydrolysis for hydrogen generation
- Growth of large gold monolayer protected-clusters
- Anion substitution reactions
- Dehydrogenation reactions
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point (°F)
-0.4 °F - closed cup
Flash Point (°C)
-18 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Regiospecific reduction of 5-benzylidene-2, 4-thiazolidinediones and 4-oxo-2-thiazolidinethiones using lithium borohydride in pyridine and tetrahydrofuran.
Giles RG
Tetrahedron, 56(26), 4531-4537 (2000)
Hui Wu et al.
Nanotechnology, 20(20), 204002-204002 (2009-05-08)
The use of Li3BN2H8 complex hydride as a practical hydrogen storage material is limited by its high desorption temperature and poor reversibility. While certain catalysts have been shown to decrease the dehydrogenation temperature, no significant improvement in reversibility has been
Mark R Biscoe et al.
Journal of the American Chemical Society, 125(42), 12718-12719 (2003-10-16)
Three hydrophobic borohydrides carrying phenyl, pentafluorophenyl, and beta-naphthyl groups were used to reduce ketones in water and in methanol. With ketones carrying phenyl, naphthyl, or biphenyl substituents, there was preferential reduction in methanol of competing acetyl groups, either intermolecular or
Sumalee Supothina et al.
The Journal of antibiotics, 57(11), 732-738 (2005-02-17)
Optimal fermentation conditions for enniatin production using the entomopathogenic fungus Verticillium hemipterigenum BCC 1449 have been investigated. Among various liquid media tested, highest efficiency of enniatin production was achieved by fermentation in yeast extract sucrose. Application of this condition to
Matthew S Wellons et al.
Nanotechnology, 20(20), 204022-204022 (2009-05-08)
The addition of catalysts to complex hydrides is aimed at enhancing the hydrogen absorption desorption properties. Here we show that the addition of carbon nanostructure C60 to LiBH4 has a remarkable catalytic effect, enhancing the uptake and release of hydrogen.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.