160954
Methyl 4-nitrobenzenesulfonate
99%
동의어(들):
Methyl nosylate, Methyl p-nitrobenzenesulfonate, Methyl p-nitrotosylate
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
O2NC6H4SO3CH3
CAS Number:
Molecular Weight:
217.20
Beilstein:
2277327
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
형태
solid
mp
89-92 °C (lit.)
solubility
acetone: soluble 5%, clear, faintly yellow to greenish-yellow
SMILES string
COS(=O)(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H7NO5S/c1-13-14(11,12)7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
InChI key
RMNJNEUWTBBZPT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Reaction between methyl 4-nitrobenzenesulfonate and bromide ions has been studied in mixed single-chain-gemini micellar solutions. Kinetics of SN2 reactions of methyl 4-nitrobenzenesulfonate with ammonia, primary amines, secondary amines, tertiary amines and anionic nucleophiles has been studied.
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
A Lewendon et al.
The Biochemical journal, 290 ( Pt 1), 15-19 (1993-02-15)
A catalytically essential histidine residue (His-195) of chloramphenicol acetyltransferase (CAT) acts as a general base in catalysis, abstracting a proton from the primary hydroxy group of chloramphenicol. The pKa of His-195 has been determined from the pH-dependence of chemical modification.
S Ishii et al.
Protein science : a publication of the Protein Society, 7(8), 1802-1810 (1999-03-19)
Aromatic L-amino acid decarboxylase (AADC) catalytic mechanism has been proposed to proceed through two consecutive intermediates (i.e., Michaelis complex and the external aldimine). Limited proteolysis of AADC that preferentially digested at the C-terminal side of Arg334 was slightly retarded in
R P Swenson et al.
The Journal of biological chemistry, 259(9), 5585-5590 (1984-05-10)
Incubation of D-amino acid oxidase with excess methyl-p-nitrobenzenesulfonate results in a pseudo-first order, irreversible loss of 95% of the assayable activity using D-phenylglycine as substrate. The rate of inactivation reaches a limiting value of 0.021 min-1 (pH 7.7, 22 degrees
María del Mar Graciani et al.
Journal of colloid and interface science, 328(2), 324-330 (2008-10-09)
The reaction between methyl 4-nitrobenzenesulfonate and bromide ions has been studied in mixed single-chain-gemini micellar solutions of n-dodecyltrimethylammonium bromide, DTAB, and dodecyl tricosaoxyethylene glycol ether, Brij(35), with alkanediyl-alpha-omega-bis(dodecyldimethylammonium) bromide, 12-s-12,2Br(-) (s=3,4,5). Kinetic micellar effects show that an increase in the
Nucleophilicity towards a saturated carbon atom: rate constants for the aminolysis of methyl 4-nitrobenzenesulfonate in aqueous solution. A comparison of the n and N+ parameters for amine nucleophilicity
Christina K M.
J. Chem. Soc. Perkin Trans. II, 11, 2291-2300 (1994)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.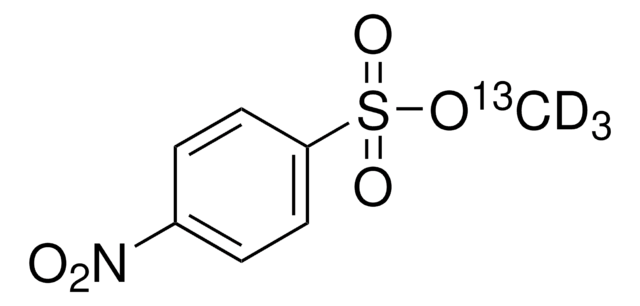






![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)


