모든 사진(1)
크기 선택
보기 변경
10 G
₩437,770
50 G
₩1,423,741
About This Item
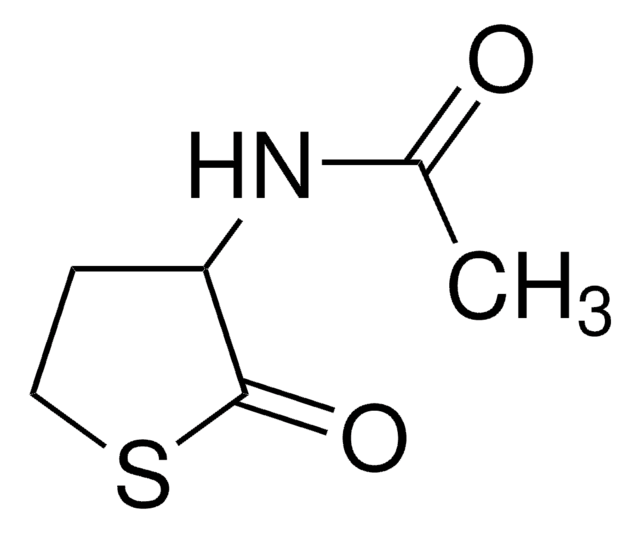
실험식(Hill 표기법):
C4H6OS
CAS Number:
Molecular Weight:
102.15
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
refractive index
n20/D 1.523 (lit.)
bp
39-40 °C/1 mmHg (lit.)
solubility
THF: soluble
density
1.18 g/mL at 25 °C (lit.)
작용기
thioester
SMILES string
O=C1CCCS1
InChI
1S/C4H6OS/c5-4-2-1-3-6-4/h1-3H2
InChI key
KMSNYNIWEORQDJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
γ-Thiobutyrolactone undergoes copolymerization with glycidyl phenyl ether to form poly(ester-alt-sulfide)[1].
애플리케이션
γ-Thiobutyrolactone was used to terminate the ring opening polymerization of ω-pentadecalactone to synthesize difunctional polyesters[2]. γ-Thiobutyrolactone was used to study the mechanism of metabolism of sulphur containing heterocyclic compounds by lignin-degrading basidiomycete Coriolus versicolor[3].
이미 열람한 고객
H Ichinose et al.
Applied microbiology and biotechnology, 58(4), 517-526 (2002-04-17)
The fungal conversions of sulfur-containing heterocyclic compounds were investigated using the lignin-degrading basidiomycete Coriolus versicolor. The fungus metabolized a series of sulfur compounds--25 structurally related thiophene derivatives--via several different pathways. Under primary metabolic conditions, C. versicolor utilized thiophenes, such as
D J Canney et al.
Bioorganic & medicinal chemistry, 6(1), 43-55 (1998-03-21)
Dihydro-2(3H)-furanones (gamma-butyrolactones) and dihydro-2(3H)-thiophenones (gamma-thiobutyrolactones) containing fluoroalkyl groups at positions C-3, C-4, and C-5 of the heterocyclic rings were prepared. The anticonvulsant/convulsant activities of the compounds were evaluated in mice. Brain concentrations of the compounds were determined and the effects
Nishikubo et al.
Macromolecules, 31(15), 4746-4752 (1998-07-29)
Poly(ester-alt-sulfide) (polymer 1) was synthesized by the alternating copolymerization of glycidyl phenyl ether (GPE) with gamma-thiobutyrolactone (TBL) catalyzed by either quaternary onium salts or crown ether complexes. The copolymerization proceeded to produce polymer 1 with good yields in neat or
K D Holland et al.
Brain research, 615(1), 170-174 (1993-06-25)
Effects of alkyl-substituted gamma-butyrolactones and gamma-thiobutyrolactones on [35S]t-butylbicyclophosphorothionate (35S-TBPS) dissociation from the picrotoxinin receptor were studied. Unlike picrotoxinin, these lactones accelerated the dissociation rate of 35S-TBPS. Thus, previous reports that these lactones change the Kd but not the Bmax of
Tiny droplets make a big splash.
Michael Eisenstein
Nature methods, 3(2), 71-71 (2006-02-14)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.