All Photos(1)
About This Item
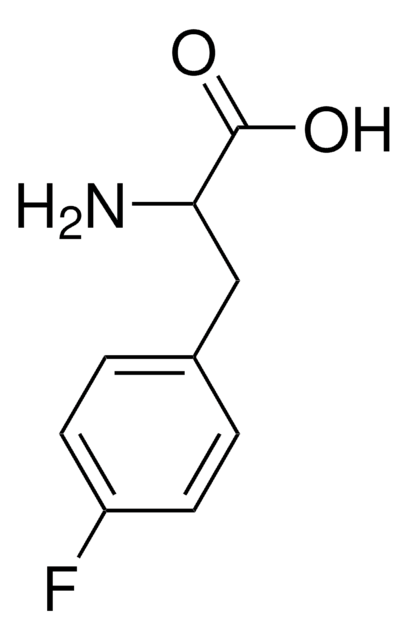
Linear Formula:
FC6H4CH2CH(NH2)COOH
CAS Number:
Molecular Weight:
183.18
Beilstein:
3201186
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95% (NT)
≥98% (HPLC)
≥98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
white to faint brown
mp
243-246 °C (lit.)
application(s)
cell analysis
peptide synthesis
SMILES string
NC(Cc1ccccc1F)C(O)=O
InChI
1S/C9H10FNO2/c10-7-4-2-1-3-6(7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
NYCRCTMDYITATC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant involved in:
- Studying mechanism of P 450-mediated oxidation and rearrangement
- Conversion of racemic α-arylalanines to (R)-β-arylalanines
- Ribosomal translation of unnatural peptides
- Synthesis of diisopropylpyridine acetamides for use as Kv1.5 potassium channel antagonists
- Enantioselective hydrolysis of esters for resolution of nonprotein amino acids
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T T Otani et al.
Journal of pharmaceutical sciences, 71(2), 214-216 (1982-02-01)
Twelve derivatives of 0-fluoro-dl-phenylalanine containing fluorine, chlorine, methoxy, and nitro radicals in various positions of the aromatic ring of the benzoyl group were prepared and tested in a Lactobacillus casei system. It was found that most substitutions in the benzoyl
H Ito et al.
Journal of nuclear medicine : official publication, Society of Nuclear Medicine, 36(7), 1232-1237 (1995-07-01)
Neutral amino acids (NAAs) are transported from the blood to the brain using the same carrier system in a competitive fashion. The purpose of this study is to establish a method for evaluating neutral amino acid transport at the blood-brain
J Hatazawa et al.
Annals of nuclear medicine, 8(3), 213-217 (1994-08-01)
We studied the brain uptake of amino acid in a patient with acute cerebral infarction with L-[2-(F-18)]fluorophenylalanine and positron emission tomography. The increased accumulation of the ligand was specifically found in the peri-infarct area where oxygen metabolism was still maintained
H Nakamichi et al.
Nuclear medicine and biology, 21(7), 959-962 (1994-10-01)
The anabolism of isotopically labeled amino acids was compared between the cerebrum and the cerebellum in conscious rat at three feeding conditions. After L-[2-18F]fluorophenylalanine and L-[2,6-3H]phenylalanine injections, the incorporation rate of both radioactivity into protein fraction showed no difference between
Elfriede Pittler et al.
Electrophoresis, 30(16), 2897-2904 (2009-08-06)
This work deals with the application of silica-based ligand-exchange chiral stationary phases (CSPs) for the enantioseparation of underivatized amino acids, alpha-hydroxy acids, and dipeptides with packed CEC. Two different possibilities of preparing silica-based CSPs are presented. One phase contains L-4-hydroxyproline
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service