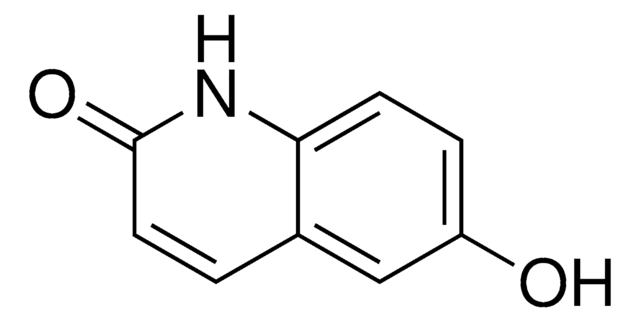
424048
2,6-Quinolinediol
Synonym(s):
2,6-Dihydroxyquinoline, 6-Hydroxycarbostyril
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
>300 °C (lit.)
SMILES string
Oc1ccc2nc(O)ccc2c1
InChI
1S/C9H7NO2/c11-7-2-3-8-6(5-7)1-4-9(12)10-8/h1-5,11H,(H,10,12)
InChI key
AQLYZDRHNHZHIS-UHFFFAOYSA-N
General description
2,6-Quinolinediol is a substituted quinoline compound. It has been prepared starting from 6-methoxyquinoline. It has been reported to be one of the UV-absorbing compounds (UAC) found in cow milk.
Application
2,6-Quinolinediol may be used as a test compound to investigate the carcinogenicity of the naturally occurring quinoline metabolite of tryptophan. The study was conducted by suspending tryptophan metabolites in cholesterol pellets, followed by the surgical implantation of the pellets into the mice bladder. It may be used as a reactant in the synthesis of 2-chloro-6-quinolinol by reacting with POCl3 (phosphorus oxychloride).
Reactant involved in synthesis of:
- (Benzyloxy)methylquinolinone derivatives for use as PDE3 inhibitors
- Substances related to cilostazol
- 2,6-Quinolinyl derivatives for use as VLA-4 / VCAM-1 antagonists
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mouse bladder carcinogenicity of certain tryptophan metabolites and other aromatic nitrogen compounds suspended in cholesterol.
Bryan GT, et al.
Cancer Research, 24(4), 596-602 (1964)
2, 6-Quinolinyl derivatives as potent VLA-4 antagonists.
Lassoie MA, et al.
Bioorganic & Medicinal Chemistry Letters, 17(1), 142-146 (2007)
In vivo elution of tryptophan metabolites and other aromatic nitrogen compounds from cholesterol pellets implanted into mouse bladders.
Bryan GT, et al.
Cancer Research, 24(4), 586-595 (1964)
Pascal Rouge et al.
Food chemistry, 141(3), 1888-1894 (2013-07-23)
The aim of this work was to characterise new UV-absorbing compounds (UAC) in cow milk in order to gain an overview of the molecular diversity of the minor bioactive constituents, that could be used to trace animal feed or that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service