410470
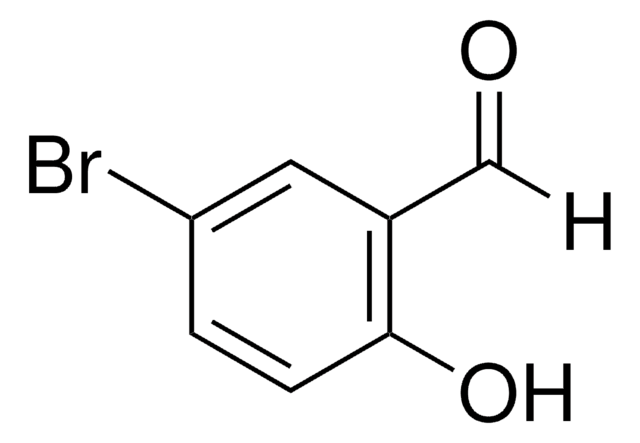
5-Bromo-2-hydroxy-3-methoxybenzaldehyde
97%
Synonym(s):
5-Bromo-3-methoxysalicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H2(OH)(OCH3)CHO
CAS Number:
Molecular Weight:
231.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
125-127 °C (lit.)
SMILES string
COc1cc(Br)cc(C=O)c1O
InChI
1S/C8H7BrO3/c1-12-7-3-6(9)2-5(4-10)8(7)11/h2-4,11H,1H3
InChI key
MMFKBTPDEVLIOR-UHFFFAOYSA-N
Related Categories
Application
5-Bromo-2-hydroxy-3-methoxybenzaldehyde may be employed for the following syntheses:
- ailanthoidol, via Stille coupling
- 6-bromo-8-methoxy-3-(methoxycarbonyl)-2H-chromen-2-one
- benzimidazole-based ligand, 2-(1H-benzoimidazol-2-yl)-4-bromo-6-methoxy-phenol (HL)
- chromogenic reagent, 5-bromo-2-hydroxy-3-methoxybenzaldehyde-p-hydroxy benzoic hydrazone
- (E)-N′-(5-bromo-2-hydroxy-3-methoxybenzylidene)-2-hydroxybenzohydrazide monohydrate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shun-Yu Lin et al.
The Journal of organic chemistry, 68(7), 2968-2971 (2003-03-29)
Ailanthoidol 1, which can be isolated from Chinese herbal medicine, is achieved in which the longest linear sequence is only six steps in 48% overall yield from commercially available 5-bromo-2-hydroxy-3-methoxybenzaldehyde. The key transformations in the synthesis are the Stille coupling
Transformation of a luminescent benzimidazole-based Yb3 cluster into a one-dimensional coordination polymer.
Yang X, et al.
Crystal Growth & Design, 10(2), 970-976 (2009)
(E)-N'-(5-Bromo-2-hydroxy-3-methoxybenzylidene)-2-hydroxybenzohydrazide monohydrate.
Zhao S, et al.
Acta Crystallographica Section E, Structure Reports Online, 68(7), 2040-2040 (2012)
Direct Spectrophotometric Determination of Ni (II) Using 5-Bromo-2-hydroxyl-3-methoxybenzaldehyde-4-hydroxy benzoichydrazone.
Saritha B and Reddy TS.
J. Appl. Chem., 7(3), 22-26 (2014)
Pedro Verdía et al.
Molecules (Basel, Switzerland), 16(6), 4379-4388 (2011-05-31)
The ionic liquid 1,3-dimethylimidazolium methyl sulfate, [MMIm][MSO₄], together with a small amount of water (the amount taken up by the ionic liquid upon exposure to air), acts efficiently as both solvent and catalyst of the Knoevenagel condensation reactions of malononitrile
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service