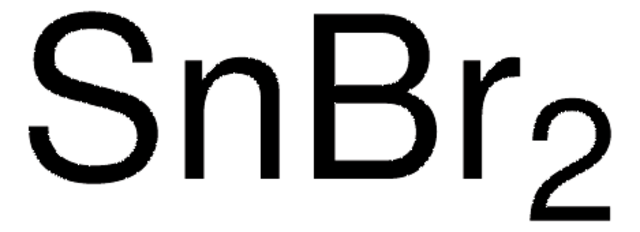409308
Tin(II) iodide
AnhydroBeads™, −10 mesh, 99.99% trace metals basis
Synonym(s):
Stannous iodide, Tin diiodide
About This Item
Recommended Products
product line
AnhydroBeads™
Assay
99.99% trace metals basis
form
beads
impurities
≤150.0 ppm Trace Metal Analysis
particle size
−10 mesh
bp
714 °C (lit.)
mp
320 °C (lit.)
density
5.28 g/mL at 25 °C (lit.)
SMILES string
I[SnH2]I
InChI
1S/2HI.Sn/h2*1H;/q;;+2/p-2
InChI key
JTDNNCYXCFHBGG-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Features and Benefits
1) Reduced uptake rate of environmental moisture minimizes caking, dusting, and static buildup for repeated easy handling.
2) Higher crucible packing densities and lower volatility in high-temperature solid state procedures.
3) Easier pneumatic loading of salts to sample chambers due to less clogging issues associated with powdered salt counterparts.
Legal Information
accessory
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 - STOT SE 3
Target Organs
Cardio-vascular system,hematopoietic system, Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service