187828
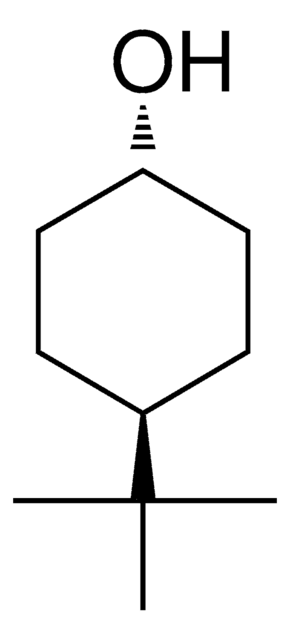
2-tert-Butylcyclohexanol, mixture of isomers
99%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CC6H10OH
CAS Number:
Molecular Weight:
156.27
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
43-46 °C (lit.)
density
0.902 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)C1CCCCC1O
InChI
1S/C10H20O/c1-10(2,3)8-6-4-5-7-9(8)11/h8-9,11H,4-7H2,1-3H3
InChI key
DLTWBMHADAJAAZ-UHFFFAOYSA-N
General description
2-tert-Butylcyclohexanol is the major metabolite of (+/-)-2-tert-butylcyclohexanone.
Application
2-tert-Butylcyclohexanol was used in the synthesis of 2-tert-butylcyclohexyl methacrylate via reaction with methacryloyl chloride in the presence of triethylamine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K L Cheo et al.
The Biochemical journal, 104(1), 198-204 (1967-07-01)
1. (+/-)-2-, (+/-)-3- and 4-tert.-Butylcyclohexanone are reduced in the rabbit to secondary alcohols, which are excreted extensively conjugated with glucuronic acid. 2. The major metabolite of (+/-)-2-tert.-butylcyclohexanone is (+)-cis-2-tert.-butylcyclohexanol, which has been isolated from the urine as [(+)-cis-2-tert.-butylcyclohexyl beta-d-glucosid]uronic acid.
Radical polymerization behavior of 2-tert-butylcyclohexyl methacrylate.
Matsumoto A and Mizuta K.
Polym. Bull., 33(2), 141-148 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service