227609
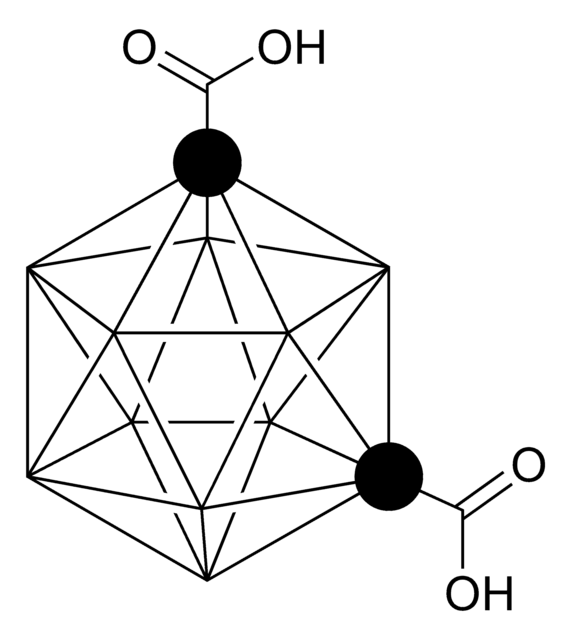
Decaborane(14)
white
Synonym(s):
Boron hydride, Decaborane, Decaboron tetradecahydride, NSC 39828, Tetradecahydrodecaborane, nido-Decaborane(14)
About This Item
Recommended Products
vapor pressure
0.15 mmHg ( 20 °C)
form
solid
reaction suitability
reagent type: reductant
color
white
bp
213 °C (lit.)
mp
98-100 °C (lit.)
density
0.94 g/mL at 25 °C (lit.)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Chemical vapor deposition of boron nitride nanosheets on Ni or Cu foils in the presence of ammonia
- Stereoselective catalytic hydrogenation reactions
- Chemical hydrogen storage
- Preparation of carborane-conjugated quinoline carboxamide ligands of translocator protein for boron neutron capture therapy (BNCT)
- Synthesis of hydrogenated boron clusters B12Hn with controlled hydrogen content
- Preparation of alkenyldecaboranes by regioselective transition-metal-catalyzed decaborane-alkyne hydroboration reactions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service