すべての画像(1)
About This Item
実験式(ヒル表記法):
C8H12N4O3
CAS番号:
分子量:
212.21
MDL番号:
UNSPSCコード:
12352200
PubChem Substance ID:
おすすめの製品
保管温度
−20°C
SMILES記法
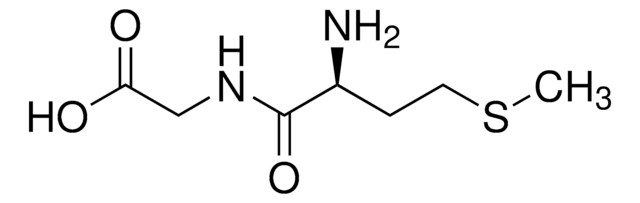
NC(Cc1cnc[nH]1)C(=O)NCC(O)=O
生物化学的/生理学的作用
Histidylleucine (His-Leu); histidylglycine (His-Gly) and histidylserine (His-Ser) are N-terminal imidazole containing dipeptides used to study binding of metals such as copper, nickel and zinc.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Anoja P Wickrama Arachchilage et al.
The Journal of chemical physics, 136(12), 124301-124301 (2012-04-03)
We have investigated the electronic structure of three cyclic dipeptides: cyclo(Histidyl-Glycyl) (cHisGly), cyclo(Tyrosyl-Prolyl) (cTyrPro), and cyclo(Phenylalanyl-Phenylalanyl) (cPhePhe) in the vapor phase, by means of photoemission spectroscopy and theoretical modeling. The last compound was evaporated from the solid linear dipeptide, but
Terézia Szabó-Plánka et al.
Inorganic chemistry, 41(13), 3483-3490 (2002-06-25)
Twelve ESR-active (and one inactive) copper(II) complexes of L-histidylglycine (HL) were characterized via their formation (micro)constants and ESR parameters obtained by two-dimensional ESR spectroscopic evaluation in aqueous solution. In strongly acidic media, the ligand is coordinated through its N-terminal donor
Brandon J Green et al.
Inorganic chemistry, 43(4), 1463-1471 (2004-02-18)
Self-decomposition of the nickel(III) doubly deprotonated peptide complex of Gly2HisGly occurs by base-assisted oxidation of the peptide. At < or =p[H+] 7.0, the major pathway is a four-electron oxidation (via 4 Ni(III) complexes) at the alpha carbon of the N-terminal
Brandon I Macdonald et al.
Rapid communications in mass spectrometry : RCM, 22(18), 2946-2954 (2008-08-30)
Pathways for proton transfer in the histidylglycine cation are examined in the gas-phase environment with the goal of understanding the mechanism by which protons may become mobile in proteins with basic amino acid residues. An extensive search of the potential
M A Hefford et al.
Biochimica et biophysica acta, 998(3), 267-270 (1989-10-19)
Secretin has a single histidine residue located at the amino terminus which plays a crucial role in its biological activity. The chemical properties, viz. pK and reactivity, of the alpha-amino and imidazole groups of this residue were determined at a
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)