おすすめの製品
由来生物
mouse
品質水準
抗体製品の状態
purified immunoglobulin
抗体製品タイプ
primary antibodies
クローン
2A3, monoclonal
化学種の反応性
mouse, rat, human, chicken
化学種の反応性(ホモロジーによる予測)
mammals (based on 100% sequence homology)
テクニック
immunocytochemistry: suitable
western blot: suitable
アイソタイプ
IgG2bκ
NCBIアクセッション番号
UniProtアクセッション番号
輸送温度
wet ice
ターゲットの翻訳後修飾
unmodified
遺伝子情報
human ... ACTG1(71)
詳細
Actin, cytoplasmic 2 (UniProt P63261; also known as Gamma-actin) is encoded by the ACTG1 (also known as ACTG, BRWS2, DFNA20, DFNA26) gene (Gene ID 71) in human. Actins are globular multi-functional proteins that serve as the basic building blocks of cytoskeletal microfilaments and are among the most conserved eukaryotic proteins. Six actin types exist, skeletal muscle alpha-actin is encoded by the ACTA1 gene, smooth muscle alpha-actin by the ACTA2 gene, cytoplasmic beta-actin by the ACTB gene, cardiac muscle alpha-actin by the ACTC1 gene, cytoplasmic gamma-actin by the ACTG1 gene, and smooth muscle gamma-actin by the ACTG2 (a.k.a. ACTA3) gene. Although actins show >90% overall sequence homology, isoforms do show spatial, temporal, and tissue-specific expression patterns and only 50-60% homology is found in their 18 N-terminal residues. Cytoplasmic β and γ-actins, are thought to be present in all cells, while the other four actin isoforms are typically found in specific adult muscle tissue types. Actins exist in a variety of structural states, depending on the specific ionic conditions or the interaction with ligand proteins. The oligomeric and polymeric forms that actin molecules assume are dependent on the distinct conformations they adopt.
特異性
Clone 2A3 detected BSA conjugated with cytoplasmic gamma-actin N-terminal peptide, but not BSA conjugated with N-terminal peptides derived from the other 5 actin types (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
免疫原
Epitope: Near N-terminus.
KLH-conjugated linear peptide corresponding to the the N-terminal sequence of human gamma-Actin/ACTG1.
アプリケーション
Research Category
細胞骨格
細胞骨格
Research Sub Category
Cytoskeleton
Cytoskeleton
This Anti-gamma-Actin/ACTG1 Antibody, clone 2A3 is validated for use in Western Blotting, Immunocytochemistry for the detection of ACTG1.
Western Blotting Analysis: 0.5 µg/mL from a representative lot detected gamma-Actin/ACTG1 in 10 µg of NIH/3T3 cell lysate.
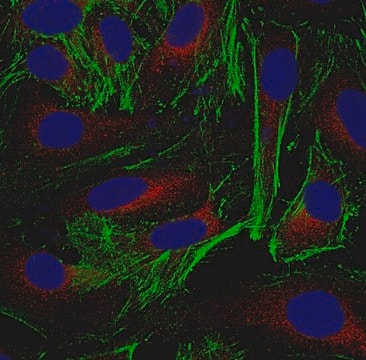
Immunocytochemistry Analysis: 5.0 µg/mL from a representative lot detected gamma-Actin/ACTG1 in HUVECs, HeLa and A431 cells.
Western Blotting Analysis: A representative lot detected downregulated cytoplasmic gamma-actin levels in stimulated (by A23187, TRAP-6, TNF, LPS, or IFN-γ) human cerebral microvascular endothelial D3 cells (hCMEC/D3) and their microparticles (MPs) when compared with unstimulated hCMEC/D3 and their MPs (Latham, S.L., et al. (2013). FASEB J. 27(2):672-683).
Western Blotting Analysis: A representative lot detected siRNA-mediated downregulation of cytoplasmic gamma-actin in A549 human lung carcinoma cells (Miazza, V., et al. (2011). Virology. 410(1):7-16).
Western Blotting Analysis: A representative lot detected cytoplasmic gamma-actin, but not beta-actin separated by 2-D gel electrophoresis of purified chicken gizzard actins or total protein extracts from human subcutaneous fibroblasts (HSCFs), canine MDCK cells, and rat aorta tissue (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
Western Blotting Analysis: A representative lot detected BSA conjugated with cytoplasmic gamma-actin N-terminal peptide, but not BSA conjugated with N-terminal peptides derived from the 5 other actin types (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
Immunocytochemistry Analysis: A representative lot detected TNF-stimulated localization of γ-actin into thick, intensely staining stress fibers concentrated apically in a submembranous network in human cerebral microvascular endothelial D3 cells (hCMEC/D3) (Latham, S.L., et al. (2013). FASEB J. 27(2):672-683).
Immunocytochemistry Analysis: A representative lot detected similar γ-actin cytoskeleton structure in human cerebral microvascular endothelial D3 cells (hCMEC/D3) with or without Rho kinase inhibitor Y-27632 treatment (Latham, S.L., et al. (2013). FASEB J. 27(2):672-683).
Immunocytochemistry Analysis: A representative lot detected a drastic subcellular redistribution of gamma-actin following Sendai virus infection of polarized Madin-Darby canine kidney (MDCK) epithelial cells by fluorescent immunocytochemistry staining of paraformaldehyde-fixed, methanol-treated cells (Miazza, V., et al. (2011). Virology. 410(1):7-16).
Immunocytochemistry Analysis: A representative lot detected cytoplasmic gamma-actin subcellular localization distinct from that of beta-actin in both spreading and stationary cells by fluorescent immunocytochemistry, using paraformaldehyde-fixed, methanol-treated HSCF human subcutaneous fibroblasts, HaCaT human keratinocytes, WI38 human embryonic fibroblasts,and Madin-Darby canine kidney (MDCK) cells (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
Immunocytochemistry Analysis: 5.0 µg/mL from a representative lot detected gamma-Actin/ACTG1 in HUVECs, HeLa and A431 cells.
Western Blotting Analysis: A representative lot detected downregulated cytoplasmic gamma-actin levels in stimulated (by A23187, TRAP-6, TNF, LPS, or IFN-γ) human cerebral microvascular endothelial D3 cells (hCMEC/D3) and their microparticles (MPs) when compared with unstimulated hCMEC/D3 and their MPs (Latham, S.L., et al. (2013). FASEB J. 27(2):672-683).
Western Blotting Analysis: A representative lot detected siRNA-mediated downregulation of cytoplasmic gamma-actin in A549 human lung carcinoma cells (Miazza, V., et al. (2011). Virology. 410(1):7-16).
Western Blotting Analysis: A representative lot detected cytoplasmic gamma-actin, but not beta-actin separated by 2-D gel electrophoresis of purified chicken gizzard actins or total protein extracts from human subcutaneous fibroblasts (HSCFs), canine MDCK cells, and rat aorta tissue (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
Western Blotting Analysis: A representative lot detected BSA conjugated with cytoplasmic gamma-actin N-terminal peptide, but not BSA conjugated with N-terminal peptides derived from the 5 other actin types (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
Immunocytochemistry Analysis: A representative lot detected TNF-stimulated localization of γ-actin into thick, intensely staining stress fibers concentrated apically in a submembranous network in human cerebral microvascular endothelial D3 cells (hCMEC/D3) (Latham, S.L., et al. (2013). FASEB J. 27(2):672-683).
Immunocytochemistry Analysis: A representative lot detected similar γ-actin cytoskeleton structure in human cerebral microvascular endothelial D3 cells (hCMEC/D3) with or without Rho kinase inhibitor Y-27632 treatment (Latham, S.L., et al. (2013). FASEB J. 27(2):672-683).
Immunocytochemistry Analysis: A representative lot detected a drastic subcellular redistribution of gamma-actin following Sendai virus infection of polarized Madin-Darby canine kidney (MDCK) epithelial cells by fluorescent immunocytochemistry staining of paraformaldehyde-fixed, methanol-treated cells (Miazza, V., et al. (2011). Virology. 410(1):7-16).
Immunocytochemistry Analysis: A representative lot detected cytoplasmic gamma-actin subcellular localization distinct from that of beta-actin in both spreading and stationary cells by fluorescent immunocytochemistry, using paraformaldehyde-fixed, methanol-treated HSCF human subcutaneous fibroblasts, HaCaT human keratinocytes, WI38 human embryonic fibroblasts,and Madin-Darby canine kidney (MDCK) cells (Dugina, V., et al. (2009). J. Cell Sci. 122(Pt 16):2980-2988).
品質
Evaluated by Western Blotting in HeLa cell lysate.
Western Blotting Analysis: 0.5 µg/mL of this antibody detected gamma-Actin/ACTG1 in 10 µg of HeLa cell lysate.
Western Blotting Analysis: 0.5 µg/mL of this antibody detected gamma-Actin/ACTG1 in 10 µg of HeLa cell lysate.
ターゲットの説明
~39-45 kDa observed. Uncharacterized band(s) may appear in some lysates.
物理的形状
Protein G Purified
Format: Purified
Purified mouse monoclonal IgG2bκ antibody in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide.
保管および安定性
Stable for 1 year at 2-8°C from date of receipt.
その他情報
Concentration: Please refer to lot specific datasheet.
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our 製品選択ツール.
保管分類コード
12 - Non Combustible Liquids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
MABT824:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Chengqing Qu et al.
Journal of the American Society of Nephrology : JASN, 33(1), 155-173 (2021-11-12)
Actin stress fibers are abundant in cultured cells, but little is known about them in vivo. In podocytes, much evidence suggests that mechanobiologic mechanisms underlie podocyte shape and adhesion in health and in injury, with structural changes to actin stress
Tomoyuki Hatano et al.
Journal of cell science, 131(8) (2018-03-15)
Actins are major eukaryotic cytoskeletal proteins, and they are involved in many important cell functions, including cell division, cell polarity, wound healing and muscle contraction. Despite obvious drawbacks, muscle actin, which is easily purified, is used extensively for biochemical studies
Rikki M Garner et al.
Cytoskeleton (Hoboken, N.J.), 77(5-6), 181-196 (2020-02-20)
Observations of actin dynamics in living cells using fluorescence microscopy have been foundational in the exploration of the mechanisms underlying cell migration. We used CRISPR/Cas9 gene editing to generate neutrophil-like HL-60 cell lines expressing GFP-β-actin from the endogenous locus (ACTB).
Kristen Skruber et al.
Current biology : CB, 30(14), 2651-2664 (2020-05-30)
Cells have many types of actin structures, which must assemble from a common monomer pool. Yet, it remains poorly understood how monomers are distributed to and shared between different filament networks. Simplified model systems suggest that monomers are limited and
Christine Chaponnier et al.
F1000Research, 5, 416-416 (2016-06-24)
Higher vertebrates (mammals and birds) express six different highly conserved actin isoforms that can be classified in three subgroups: 1) sarcomeric actins, α-skeletal (α-SKA) and α-cardiac (α-CAA), 2) smooth muscle actins (SMAs), α-SMA and γ-SMA, and 3) cytoplasmic actins (CYAs)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)