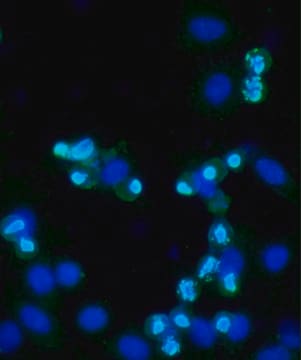
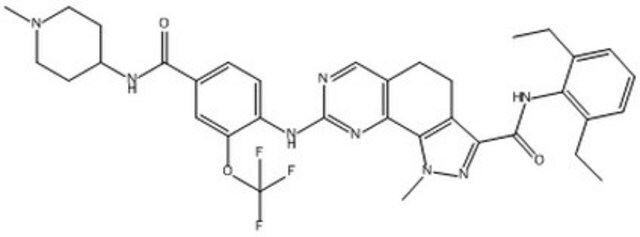
An orally bioavailable, ATP-competitive, pyrazolo-quinazoline, MPS1 inhibitor (IC
50 = 182 nM, K
i = 0.99 nM) that is shown to act in a reversible and time-dependent manner. It demonstrates selectivity for MPS1 against a panel of 60 kinases, displaying activity against only three kinases, CK2, MELK, and NEK6 (<10 M), but not against other mitotic kinases including PLK1, CDK1, Aurora A, Aurora B, or the SAC kinase BUB1, in an
in vitro kinase assay. It promotes massive SAC (spindle assembly checkpoint) override (EC
50 = 65 nM) in nocodazole-arrested U20S cells and elicits a reduction in the G1 and G2/M phase of the cell cycle in A2780 ovarian cancer cells, similar to RNAi-mediated MPS1 silencing. In addition, it is shown to inactivate SAC, delocalize kinetochore components, and inhibit the proliferation of select cancer cell lines (IC
50 ~ 1 M), without marked activity among a panel of 127 normal cell lines. Also, it inhibits A2780 tumor xenograft growth in mice (90 mg/kg/day, o.s.,
in vivo) by 53% without marked body weight loss. Also available as a 5 mM solution in DMSO (Cat. No.
506313).
An orally bioavailable, ATP-competitive, pyrazolo-quinazoline, MPS1 inhibitor (IC50 = 182 nM, Ki = 0.99 nM) that is shown to act in a reversible and time-dependent manner. It demonstrates selectivity for MPS1 against a panel of 60 kinases, displaying activity against only three kinases, CK2, MELK, and NEK6 (<10 µM), but not against other mitotic kinases including PLK1, CDK1, Aurora A, Aurora B, or the SAC kinase BUB1, in an in vitro kinase assay. It promotes massive SAC (spindle assembly checkpoint) override (EC50 = 65 nM) in nocodazole-arrested U20S cells and elicits a reduction in the G1 and G2/M phase of the cell cycle in A2780 ovarian cancer cells, similar to RNAi-mediated MPS1 silencing. In addition, it is shown to inactivate SAC, delocalize kinetochore components, and inhibit the proliferation of select cancer cell lines (IC50 ~ 1 µM), without marked activity among a panel of 127 normal cell lines. Also, it inhibits A2780 tumor xenograft growth in mice (90 mg/kg/day, o.s., in vivo) by 53% without marked body weight loss.