444290
MMP Inhibitor V
The MMP Inhibitor V, also referenced under CAS 223472-31-9, controls the biological activity of MMP. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
別名:
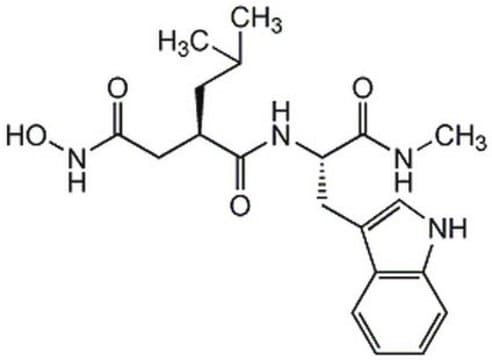
MMP Inhibitor V, (2S,4S)-N-Hydroxy-5-ethoxymethyloxy-2-methyl-4-(4-phenoxybenzoyl)aminopentanamide, ONO-4817
About This Item
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
off-white
溶解性
ethanol: 15 mg/mL
DMSO: 30 mg/mL
輸送温度
ambient
保管温度
2-8°C
InChI
1S/C22H28N2O6/c1-3-28-15-29-14-18(13-16(2)21(25)24-27)23-22(26)17-9-11-20(12-10-17)30-19-7-5-4-6-8-19/h4-12,16,18,27H,3,13-15H2,1-2H3,(H,23,26)(H,24,25)/t16-,18-/m0/s1
InChI Key
HDWWQELUBWGQGA-WMZOPIPTSA-N
詳細
包装
警告
その他情報
Ito, N., et al. 2004. Biochem. Biophys. Res. Commun.314, 1008.
Shiraga, M., et al. 2002. Cancer Res.62, 5967.
Mori, T., et al. 2001. Exp. Biol. Med.226, 429.
Yamada, A., et al. 2000. Inflamm. Res.49, 144.
法的情報
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
444290-MG:
444290-2MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
関連コンテンツ
Select different protease inhibitor types based on your needs to prevent protein degradation during isolation and characterization and safeguard proteins in sample prep.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)