Custom DNA Immunoprescription Rat / Mouse Monoclonal Antibody Production
Special vectors inserted with the target gene are used to immunize animals to produce antibodies. Our method enables the production of antibodies against challenging targets in conventional methods or those that can be used for advanced applications.
- Our unique DNA immunization and iliac lymph node method are combined to produce rat/mouse monoclonal antibodies.
- Antibodies against challenging targets in conventional methods, such as multiple transmembrane proteins, can be produced in a highly efficient way.
- Dedicated antibody experts who have produced hundreds of antibodies provide full support for the production of your desired antibody.
- Intellectual property rights for the custom antibody will be completely transferred to the customer upon delivery. The customer can freely patent, develop, distribute, and transfer the custom antibody, with no license-related barriers to commercialization.
Technologies for rat/mouse monoclonal antibody production by DNA immunization
DNA Immunoprescription
We have developed our original immune vector. The vectors inserted with the full-length cDNA of a target protein are used to immunize mice or rats by electroporation. The vectors induce the co-expression of target proteins and immune activation factors in immunized animals to elicit antibody production. By using this method eliminating the need for preparing a large amount of antigen for immunization, you can immunize even with a target that is difficult to prepare.
Iliac Lymph Node Method
Various antibodies to different epitopes can be obtained using lymphocytes from the iliac lymph node. Because the positive rate is overwhelmingly higher than that with the spleen method, the Iliac lymph node method can increase the probability of obtaining antibodies with higher titer, affinity, and specificity.

Anti-Membrane Protein Antibody
By combining DNA immunization and the iliac lymph node method, antibodies against multiple transmembrane proteins that had been extremely difficult to prepare can be produced in a highly efficient way. This is good news for researchers studying multiple transmembrane proteins, single transmembrane proteins, and extracellularly presented intracellular proteins. This method is also recommended for targets for which preparation of recombinant proteins is difficult, such as virus-derived proteins.
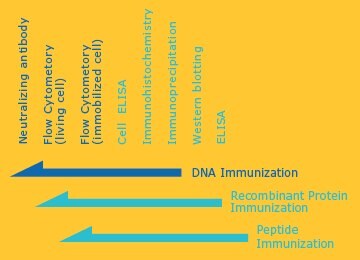
Antibodies that can be Used for Various Applications
Antibodies can be used for advanced applications, such as flow cytometric analysis of live cells, cell ELISA, immune cell staining, and functional inhibition.

Customer-Initiated Work Testing of Antibodies
After flow site-based screening of cells coexpressing target protein and GFP, culture supernatant of positive clones will be provided to the customer for evaluation of whether the antibodies actually work in the customer's application.
Also, the entire process will be divided into 6 steps, and for each step, the customer will decide whether or not to proceed to the next step. Billing will occur at the end of each step.
PROCEDURE FOR RAT/MOUSE MONOCLONAL ANTIBODY PRODUCTION BY DNA IMMUNIZATION
Step 1
- Construction of target protein forced expression plasmid and work checking
Deliverables
- Work report (cell immunostaining data for forced-expressing cells)
Step 2
- DNA immunization with the immunization plasmid (mouse or rat)
- Check for titer after 8 weeks of immunization
Deliverables
- Work report (FCM data evaluating individual sera)
Step 3
- Boost immunization
- Cell fusion (fusion of iliac lymph node lymphocytes and splenocytes with myeloma cells)
- Seeding in 96-well plates
Deliverables
- Work report (Lymph node enlargement and the target number of plate implants will be guaranteed)
Step 4
- 1st screening
- Temporal freezing of hybridomas in positive wells (during customer evaluation)
Deliverables
- Screening data
- Positive culture supernatant samples
Customer Work Test (Evaluation and Judgement)
Step 5
- Cloning
- 2nd screening
- Temporary storage of hybridomas (under customer evaluation)
Deliverables
- Screening data
- Positive culture supernatant sample
Customer Work Test (Evaluation and Judgement)
Step 6
- Hybridoma scale-up
- Isotyping
Deliverables
- Hybridomas
As a result of the hearing by our engineer, we may suggest a process different from above.
The customer will decide whether or not to proceed to the next step at completion of each step. After completion of each step, an operation fee will be charged. At the evaluation phase after step 5, if a satisfactory antibody is obtained, we will proceed to step 6 to deliver the clone.
A maximum of 3 wells can be selected in step 4 and up to 3 clones in step 5 at no additional charge.
Customer work test should be completed within approximately 4 weeks.
Flow from ordering to delivery
Based on information obtained from the inquiries, a dedicated antibody expert will have a technical meeting with the customer to prepare the desired antibody.
A work schedule for the production of the antibody will be prepared and shown with the fee, and the customer can place the order if satisfied. A full technical support system will be in place during the process to provide progress reports and address requests for additional works.
1. Contact
*Account registration is required to place an order. Register here.
2. Primary hearing
A technical service person will ask about the information and requests regarding the desired antibody to be produced.
3. Final meeting
An engineer will visit the customer with the service person to discuss details such as the work process and goal settings.
4. Order
Based on the content of the meeting, we will provide a schedule and an estimate, and the customer can place the order if satisfied.
5. Antibody preparation work
Steps 1 to 6
(plasmid construction, DNA immunization, isolation of iliac lymph node lymphocytes and spleen cells, hybridoma preparation, and screening)
6. Delivery of materials
Hybridomas
Related Product Resources
Article: Custom monoclonal antibody production: Frequently asked questions (FAQs)
Customer Service
To continue reading please sign in or create an account.
Don't Have An Account?