17304
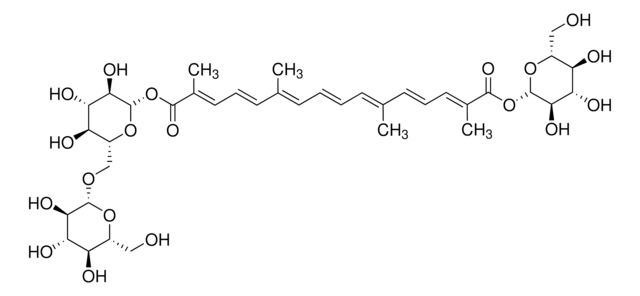
Crocin
for microscopy
Synonym(s):
Crocetin digentiobiose ester
About This Item
Recommended Products
grade
for microscopy
form
solid
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
CC(=C\C=C\C=C(C)\C=C\C=C(/C)C(=O)O[C@@H]1O[C@H](CO[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@H](O)[C@H]1O)\C=C\C=C(\C)C(=C)CO[C@@H]3O[C@H](CO[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)[C@@H](O)[C@H](O)[C@H]3O
InChI
1S/C46H68O23/c1-21(12-8-14-23(3)25(5)18-62-43-40(59)36(55)32(51)28(67-43)19-63-44-38(57)34(53)30(49)26(16-47)65-44)10-6-7-11-22(2)13-9-15-24(4)42(61)69-46-41(60)37(56)33(52)29(68-46)20-64-45-39(58)35(54)31(50)27(17-48)66-45/h6-15,26-41,43-60H,5,16-20H2,1-4H3/b7-6+,12-8+,13-9+,21-10-,22-11+,23-14-,24-15+/t26-,27-,28-,29-,30-,31-,32-,33-,34+,35+,36+,37+,38-,39-,40-,41-,43-,44-,45-,46+/m1/s1
InChI key
LUVDBMJRTUBHNX-RSXXRLSLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a reference standard to detect its presence in Crocus sativus L. extract using high-performance liquid chromatography technique
- to investigate its protective effects as an antioxidant and anti-inflammatory agent against doxorubicin-induced nephrotoxicity in rats
- to test antitumor effects and its role in promoting autophagy and apoptosis and inhibiting the progression of cervical cancer on SiHa cells and female BALB/c nude mice
- as a co-treatment with cisplatin to test its protective effect against cisplatin (CIS)-induced testicular toxicity in rats
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
17304-BULK:
17304-1G:
17304-5G:
17304-VAR:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service