208523
Ruthenium(III) chloride
Ru content 45-55%
Synonym(s):
Ruthenium trichloride
About This Item
Recommended Products
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Atom Transfer Radical Polymerization (ATRP)
density
3.11 g/mL at 25 °C (lit.)
SMILES string
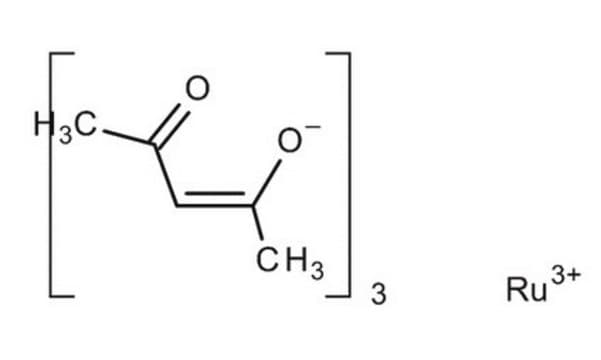
Cl[Ru](Cl)Cl
InChI
1S/3ClH.Ru/h3*1H;/q;;;+3/p-3
InChI key
YBCAZPLXEGKKFM-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In the synthesis of β‐amino alcohols by nucleophilic opening of epoxides with anilines.
- In the acetylation of varies of phenols, alcohols, thiols, and amines under mild conditions.
- In the synthesis of α‐aminonitriles by mixing aldehydes, amines, and trimethylsilyl cyanides.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
208523-10G:
208523-VAR:
208523-50G:
208523-BULK:
208523-2G:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization.
Protocols
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Polymerization via ATRP procedures demonstrated by Prof. Dave Haddleton's research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service