700080P
Avanti
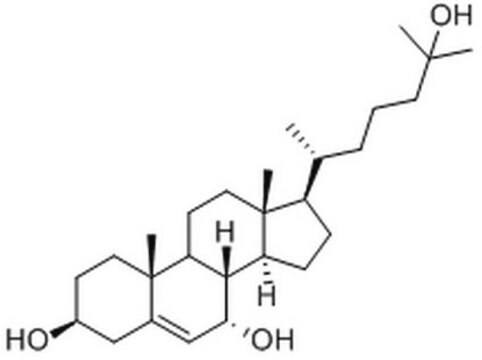
7α,25-dihydroxycholesterol
Avanti Research™ - A Croda Brand
Synonym(s):
cholest-5-ene-3β,7α,25-triol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H46O3
CAS Number:
Molecular Weight:
418.65
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 1 mg (700080P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand
shipped in
dry ice
storage temp.
−20°C
General description
7α,25-dihydroxycholesterol (7α,25-OHC) is synthesized by the hydroxylation of cholesterol by the action of enzyme cholesterol 25-hydroxylase (CH25H) and cytochrome P450, family 7, subfamily b, polypeptide 1 (CYP7B1). The catabolic breakdown of 7α,25-OHC to bile acid precursors is catalyzed by the enzymes hydroxy-Δ-5-steroid dehydrogenase, 3β- and steroid Δ-isomerase 7 (HSD3B7).
Application
7α,25-dihydroxycholesterol has been used:
- as a G-protein-coupled receptor 183 ( GPR183) ligand in chemotaxis assay and intracellular cytokine staining method and in fluorescence-activated cell sorting (FACS) analysis of natural killer cells
- in oxysterol based calcium mobilization assay in Chinese hamster ovary (CHO) cells
- in competitive radioligand binding assay of Epstein-Barr virus-induced gene 2 (EBI2) expressing COS-7 cells
Biochem/physiol Actions
7α,25-dihydroxycholesterol (7α,25-OHC) serves as an endogenous ligand for G-protein-coupled receptor 183 (GPR183) or Epstein-Barr virus-induced gene 2 (EBI2). It plays a key role in the migration of type 3 innate lymphoid cells (ILC3) in the small intestine and colon. 7α,25-OHC mediates immune cell migration functionality by their chemoattractant property. Inhibition of 7α,25-OHC synthesis leads to impairment in the migration of B cells.
Packaging
5 mL Amber Glass Screw Cap Vial (700080P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
also commonly purchased with this product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gene regulatory programs conferring phenotypic identities to human NK cells
Collins PL, et al.
Cell, 176(1-2), 348-360 (2019)
Identification of structural motifs critical for epstein-barr virus-induced molecule 2 function and homology modeling of the ligand docking site
Zhang L, et al.
Molecular Pharmacology, 82(6), 1094-1103 (2012)
Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation
Emgaard J, et al.
Immunity, 48(1), 120-132 (2018)
Katharina R Beck et al.
The Journal of steroid biochemistry and molecular biology, 190, 19-28 (2019-03-25)
Oxysterols are cholesterol metabolites derived through either autoxidation or enzymatic processes. They consist of a large family of bioactive lipids that have been associated with the progression of multiple pathologies. In order to unravel (patho-)physiological mechanisms involving oxysterols, it is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service