PHL89593
Camptothecin
phyproof® Reference Substance
Sinonimo/i:
(S)-(+)-Camptothecin
About This Item
Prodotti consigliati
Grado
primary reference standard
Nome Commerciale
phyproof® Reference Substance
Saggio
≥90.0% (HPLC)
Forma fisica
powder
Produttore/marchio commerciale
PhytoLab
Punto di fusione
260 °C (dec.) (lit.)
Temperatura di conservazione
2-8°C
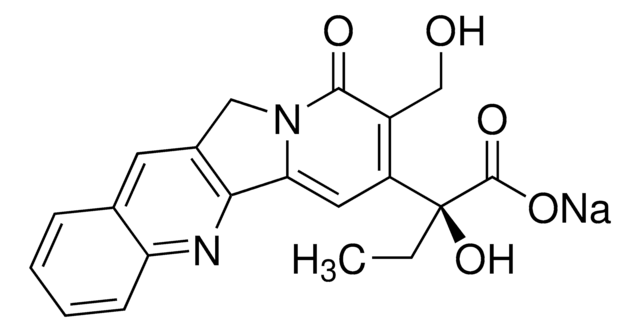
Stringa SMILE
CC[C@@]1(O)C(=O)OCC2=C1C=C3N(Cc4cc5ccccc5nc34)C2=O
InChI
1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
VSJKWCGYPAHWDS-FQEVSTJZSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Co-delivery system for camptothecin and doxorubicin: Research on dendritic polymer prodrug-based unimolecular micelles demonstrated a pH-responsive co-delivery mechanism for camptothecin and doxorubicin, offering a synergistic effect in controlled drug release (Chen and Liu, 2024).
- Investigation into camptothecin′s role in chronic myeloid leukemia: The study explored the therapeutic potential of FL118, a camptothecin derivative, against chronic myeloid leukemia resistant to BCR-ABL inhibitors, targeting RNA helicase DDX5 (Takeda et al., 2024).
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Muta. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.