BCR158
Benz[c]acridine
BCR®, certified reference material
About This Item
Prodotti consigliati
Grado
certified reference material
agenzia
BCR®
Produttore/marchio commerciale
JRC
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
c1ccc2nc3c(ccc4ccccc34)cc2c1
InChI
1S/C17H11N/c1-3-7-15-12(5-1)9-10-14-11-13-6-2-4-8-16(13)18-17(14)15/h1-11H
OAPPEBNXKAKQGS-UHFFFAOYSA-N
Risultati analitici
BCR158
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.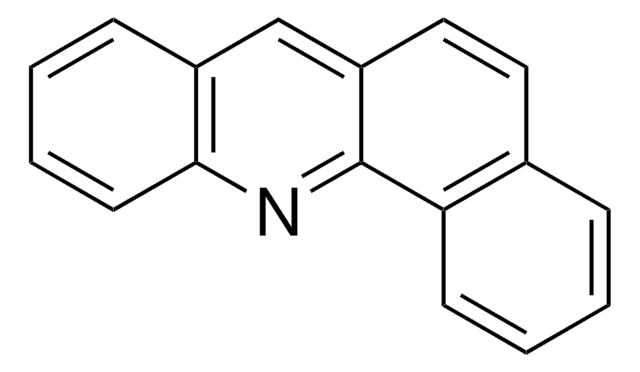
![12-chlorobenzo[b]acridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/247/340/8c0d93cd-830f-4d5f-b29f-8b154b59ac25/640/8c0d93cd-830f-4d5f-b29f-8b154b59ac25.png)

![Benz[a]acridine BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/207/419/0aadf1df-8fad-4c85-b2cc-73ba0e6fa8b4/640/0aadf1df-8fad-4c85-b2cc-73ba0e6fa8b4.png)
![4H-benzo[def]carbazole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/319/198/4208123a-456b-4f27-bc62-bc3af7c1d403/640/4208123a-456b-4f27-bc62-bc3af7c1d403.png)