CC1048
MMP-9, human, proform
Sinonimo/i:
Progelatinase B, 92 kDa Type IV Collagenase
About This Item
Prodotti consigliati
Origine biologica
human
Saggio
>95% (total protein)
Forma fisica
liquid
Attività specifica
>1200 mU/mg (specific activity of activated MMP-9 after trypsin activation)
Produttore/marchio commerciale
Chemicon®
Concentrazione
0.2 mg/mL
N° accesso NCBI
N° accesso UniProt
Condizioni di spedizione
dry ice
Informazioni sul gene
human ... MMP9(4318)
Descrizione generale
Applicazioni
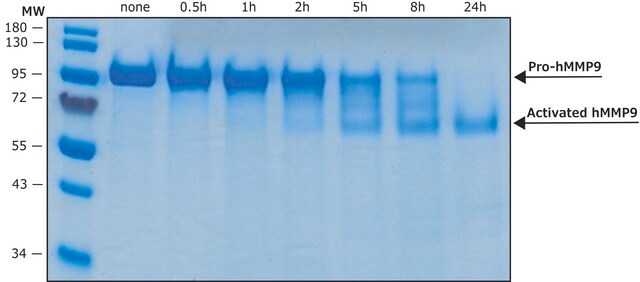
An aliquot of 10 μL MMP-9 monomer is mixed with 20 μL trypsin solution (see below) and activation buffer in a total volume of 100 μL. The mixture is incubated for 20 min at 37°C. Thereafter trypsin is inhibited by addition of 10 μL aprotinin solution.
Trypsin solution: 0.50 mg TPCK-trypsin/mL activation buffer. The solution is stored in aliquots at -20°C.
Activation Buffer: 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2.
Aprotinin solution: 1 mg aprotinin/mL activation buffer. The solution is stored at -20°C.
Definizione di unità
Stato fisico
Stoccaggio e stabilità
Risultati analitici
Note legali
Esclusione di responsabilità
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.