G10806
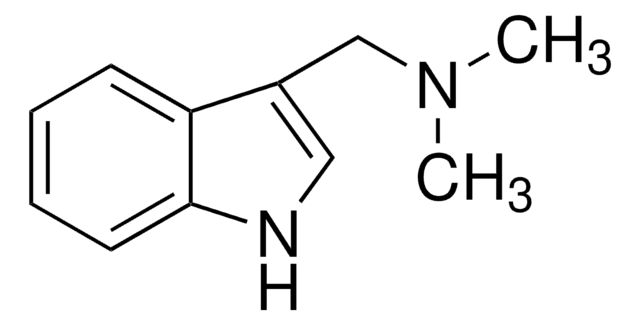
Gramine
97.5%
Sinonimo/i:
3-(Dimethylaminomethyl)indole, Donaxine, NSC 16892
About This Item
Prodotti consigliati
Saggio
97.5%
Punto di fusione
132-134 °C (lit.)
Stringa SMILE
CN(C)Cc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-13(2)8-9-7-12-11-6-4-3-5-10(9)11/h3-7,12H,8H2,1-2H3
OCDGBSUVYYVKQZ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Dopamine D2 receptor antagonists
- Anti-malarial drugs
- 5-indolyl-Mannich bases
- Proliferation inhibitors
- Inhibitors of human mast cell chymase
- Preparation of DL-tryptophan
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- 3-vinylindoles
- Serotonin 5-HT6 receptor ligand templates
- Selective protein kinase c delta (PKCδ) down regulators
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
332.6 °F
Punto d’infiammabilità (°C)
167 °C
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.