D12600
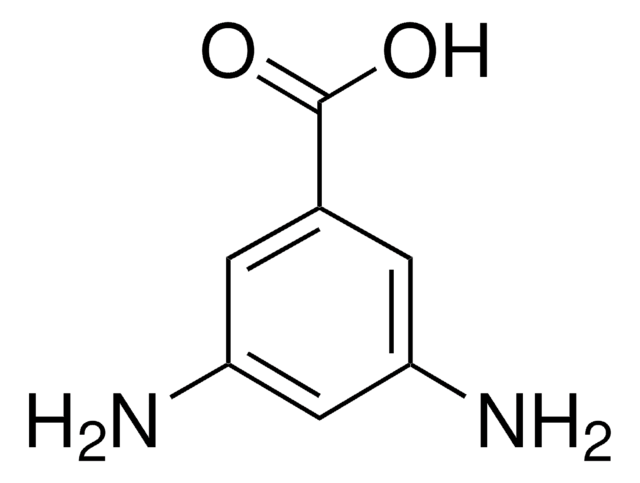
3,4-Diaminobenzoic acid
97%
Sinonimo/i:
4-Carboxy-o-phenylenediamine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(H2N)2C6H3CO2H
Numero CAS:
Peso molecolare:
152.15
Beilstein:
775892
Numero CE:
Numero MDL:
Codice UNSPSC:
12352200
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
powder
Impiego in reazioni chimiche
reaction type: solution phase peptide synthesis
Punto di fusione
208-210 °C (dec.) (lit.)
applicazioni
peptide synthesis
Stringa SMILE
Nc1ccc(cc1N)C(O)=O
InChI
1S/C7H8N2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3H,8-9H2,(H,10,11)
HEMGYNNCNNODNX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
3,4-Diaminobenzoic acid can be used to prepare:
- Schiff base derivatives by reacting with substituted aldehydes and their corresponding metal complexes.
- Poly(2,5-benzimidazole) (ABPBI) polymer by reacting with methanesulfonic acid and P2O5.
- Pt-based Schiff base complexes applicable in H2O splitting reactions.
3,4-Diaminobenzoic acid undergoes cyclocondensations to form, for example, quinoxalines and benzimidazoles.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
N Sundaraganesan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 70(2), 376-383 (2007-12-28)
The Fourier Transform Raman and Fourier Transform infrared spectra of 3,4-diaminobenzoic acid (3,4-DABA) were recorded in the solid phase. Geometry optimizations were done without any constraint and harmonic-vibrational wave numbers and several thermodynamic parameters were calculated for the minimum energy
New platinum and ruthenium Schiff base complexes for water splitting reactions
Wang C, et al.
Dalton Transactions, 44(32), 14483-14493 (2015)
L A Cooper et al.
Antonie van Leeuwenhoek, 50(1), 53-62 (1984-01-01)
The effect of various compounds on growth, melanin biosynthesis and cell differentiation was studied in a hyaline (SH25) and a pigmented (SH25B) strain of Microdochium bolleyi. Dark pigment production by the hyaline strain was induced by the presence of DOPA
S Ram et al.
Journal of medicinal chemistry, 35(3), 539-547 (1992-02-07)
A series of methyl and ethyl 5-(alkoxycarbonyl)-1H-benzimidazole-2-carbamates (7-19) and methyl 5-carbamoyl-1H-benzimidazole-2-carbamates (24-34) have been synthesized via the reaction of an appropriate alcohol or amine with the acid chloride derivatives 6a or 6b at room temperature. Reaction of an alcohol with
Tetrahedron, 49, 9823-9823 (1993)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.