939358
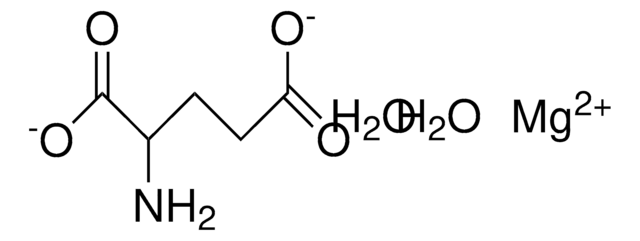
Magnesium acetate tetrahydrate

≥99.9% trace metals basis
Sinonimo/i:
Magnesium Diacetate Tetrahydrate,, Magnesium diethanoate tetrahydrate, Acetic acid magnesium salt
About This Item
Prodotti consigliati
Tipo
(High purity salts)
Livello qualitativo
Saggio
≥99.9% trace metals basis
98-102% (EDTA, complexometric)
Forma fisica
powder or crystals
solid
Impurezze
<1000 ppm trace metal basis
Colore
white to off-white
Punto di fusione
72-75 °C (lit.)
72-75 °C
Solubilità
water: soluble
Densità
1.454 g/cm3
Anioni in tracce
chloride (Cl-): ≤20 ppm
sulfate (SO42-): <50 ppm
Cationi in tracce
Al: <50 ppm
K: <50 ppm
Mg: <100 ppm
Na: <50 ppm
Pb: <50 ppm
Zn: <50 ppm
applicazioni
battery manufacturing
Stringa SMILE
O.O.O.O.CC(=O)O[Mg]OC(C)=O
InChI
1S/2C2H4O2.Mg.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
XKPKPGCRSHFTKM-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- A key components for the synthesis of spinel magnesium manganese oxide (Mg0.5Mn2.5O4 ) through one-step colloidal synthesis method. The nanocrystals has exhibited significant electrochemical activities in presence of diverse electrolytes.
- A starting materials for the production of Mesoporous (ZnO)x(MgO)1−x nanoplates by a template-free solvothermal synthetic method followed by subsequent calcination. These materials exhibit a band gap resulting from the presence of ZnO and MgO. The broad peaks observed in the 400-700 nm range indicate the presence of oxygen vacancy defects on the surface of the (ZnO)x(MgO)1−x nanoplates. These nanocrystals showed superior photocatalytic activities for the degradation of methyl orange (MO) in aqueous solution.
- To the synthesis of hydrophobic antireflective films of MgF2 with silicon modified with enhenced durability through sol-gel method.
- As a material for synthesizing carbon nanoribbons using ferrocene at high temperatures. The resulting nanoribbons exhibit a remarkably high surface area and demonstrate a stable reversible capacity of 750 mA h g−1 after 300 cycles in a charge-discharge experiment conducted at 0.5 A g−1. Due to these properties, it would be highly beneficial as an electrode material in electronic devices.
Caratteristiche e vantaggi
Medium purity (99.9%)
Low trace metals in ppm level
Cost effective
Low Chloride and sulfate levels
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.