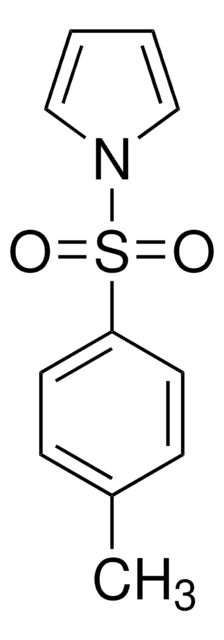
438839
1-(Phenylsulfonyl)pyrrole
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H9NO2S
Numero CAS:
Peso molecolare:
207.25
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
solid
Punto di fusione
88-91 °C (lit.)
Stringa SMILE
O=S(=O)(c1ccccc1)n2cccc2
InChI
1S/C10H9NO2S/c12-14(13,11-8-4-5-9-11)10-6-2-1-3-7-10/h1-9H
PPPXRIUHKCOOMU-UHFFFAOYSA-N
Descrizione generale
1-(Phenylsulfonyl)pyrrole is a heterocyclic building block. 1-(Phenylsulfonyl) group serves as N-blocking and directing group in various organic syntheses.
Applicazioni
1-(Phenylsulfonyl)pyrrole (1-phenylsulfonyl-1H-pyrrole) may be used in the synthesis of 1-(phenylsulfonyl)pyrrole-2-boronic acid, via lithiation of 1-(phenylsulfonyl)-pyrrole. It may be used for the synthesis of 1-phenylsulfonyl-1H-pyrrole-3-sulfonyl chloride derivatives, which affords sulfonamide derivatives by reaction with nitrogen nucleophiles.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Efficient sulfonation of 1-phenylsulfonyl-1H-pyrroles and 1-phenylsulfonyl-1H-indoles using chlorosulfonic acid in acetonitrile.
Janosik T, et al.
Tetrahedron, 62(8), 1669-1707 (2006)
Pyrrole chemistry. XXVIII. Substitution reactions of 1-(phenylsulfonyl) pyrrole and some derivatives.
Anderson HJ, et al.
Canadian Journal of Chemistry, 63(4), 896-902 (1985)
Synthesis of 2-Aryl-1-(phenylsulfonyl) pyrroles.
Grieb JG and Ketcha DM.
Synthetic Communications, 25(14), 2145-2153 (1995)
Papireddy Kancharla et al.
The Journal of organic chemistry, 79(23), 11674-11689 (2014-11-08)
Facile and highly efficient synthetic routes for the synthesis of (S)- and (R)-23-hydroxyundecylprodiginines ((23S)-2, and (23R)-2), 23-ketoundecylprodiginine (3), and deuterium-labeled 23-hydroxyundecylprodiginine ([23-d]-2) have been developed. We demonstrated a novel Rieske oxygenase MarG catalyzed stereoselective bicyclization of (23S)-2 to premarineosin A
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.