273937
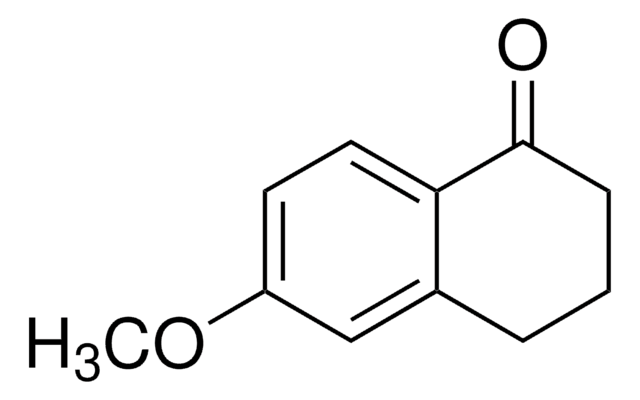
6,7-Dimethoxy-1-tetralone
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C12H14O3
Numero CAS:
Peso molecolare:
206.24
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Forma fisica
powder
Punto di fusione
98-100 °C (lit.)
Stringa SMILE
COc1cc2CCCC(=O)c2cc1OC
InChI
1S/C12H14O3/c1-14-11-6-8-4-3-5-10(13)9(8)7-12(11)15-2/h6-7H,3-5H2,1-2H3
YNNJHKOXXBIJKK-UHFFFAOYSA-N
Descrizione generale
6,7-Dimethoxy-1-tetralone reacts with 2-amino-4,5-dimethoxyacetophenone to form 5,6-dihydro-2,3,9,10-tetramethoxybenz[c]acridine.
Applicazioni
6,7-Dimethoxy-1-tetralone was used in the synthesis of 2-bromotetralones by undergoing bromination. It was also used as a precursor to quinolines with dopaminergic activity, naphthols with anti-inflammatory activity and benzophenanthridine alkaloids with antitumor activity.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
The Journal of Organic Chemistry, 57, 5907-5907 (1992)
Colin J Dunsmore et al.
Bioorganic & medicinal chemistry letters, 18(5), 1730-1734 (2008-02-12)
Several 2-aminotetralones were identified as novel inhibitors of the bacterial enzymes MurA and MurZ. A number of these inhibitors demonstrated antibacterial activity against Staphylococcus aureus and Escherichia coli with MICs in the range 8-128 microg/ml. Based on structure-activity relationships we
J C Craig et al.
Journal of medicinal chemistry, 32(5), 961-968 (1989-05-01)
A series of 2-substituted octahydrobenzo[f]quinolines has been synthesized and assayed for dopamine agonist activity. Only the compounds corresponding to the beta-rotameric conformation of dopamine showed biphasic activity in competition binding studies with the radioligand [3H]spiroperidol. These findings suggest that the
D G Batt et al.
Journal of medicinal chemistry, 33(1), 360-370 (1990-01-01)
The synthesis, biological evaluation, and structure-activity relationships of a series of 1-naphthols bearing carbon substituents at the 2-position are described. These compounds are potent inhibitors of the 5-lipoxygenase from RBL-1 cells and also inhibit bovine seminal vesicle cyclooxygenase. Structure-activity relationships
D Makhey et al.
Bioorganic & medicinal chemistry, 8(5), 1171-1182 (2000-07-06)
Coralyne and several other synthetic benzo[a,g]quinolizium derivatives related to protoberberine alkaloids have exhibited activity as topoisomerase poisons. These compounds are characterized by the presence of a positively charged iminium group, which has been postulated to be associated with their pharmacological
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.