36849
Fenoxaprop
PESTANAL®, analytical standard
Synonym(s):
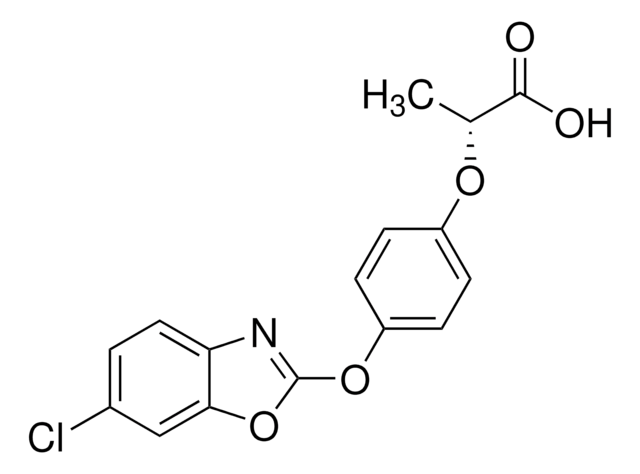
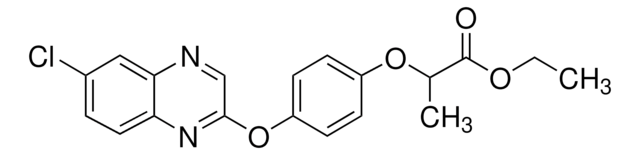
2-{4-[(6-Chlorobenzoxazol-2-yl)oxy]phenoxy}propionic acid
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
storage temp.
2-8°C
SMILES string
CC(Oc1ccc(Oc2nc3ccc(Cl)cc3o2)cc1)C(O)=O
InChI
1S/C16H12ClNO5/c1-9(15(19)20)21-11-3-5-12(6-4-11)22-16-18-13-7-2-10(17)8-14(13)23-16/h2-9H,1H3,(H,19,20)
InChI key
MPPOHAUSNPTFAJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
According to Commission Regulation (1107/2009), fenoxaprop is not approved for use as a plant protection product in the European Union. However, a default MRL of 0.01 mg/kg is set according to Art 18(1)(b) Reg 396 / 2005.
Application
- Develop an HPLC method for the determination of fenoxaprop-ethyl and fenoxaprop residues in four soil types using two extraction procedures
- Validate the resistance of fenoxaprop in wild oats in Turkey and investigate cross and multiple resistance patterns of fenoxaprop-resistant wild oat populations
- Investigate the degradation of fenoxaprop-ethyl and fenoxaprop in three soils were under native and sterilized conditions using enantioselective high-performance liquid chromatography (HPLC)
- Determine the effect of soil moisture, temperature, and light intensity on the spray deposition of fenoxaprop and imazamethabenz applied to wild oat plants
- Investigate and quantify the resistance of Japanese foxtail (Alopecurus japonicus) to fenoxaprop and pinoxaden in China and elucidate the basis of resistance to these herbicides
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service