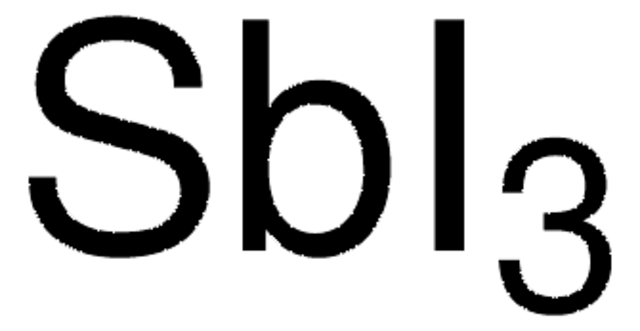All Photos(2)
About This Item
Empirical Formula (Hill Notation):
AgI
CAS Number:
Molecular Weight:
234.77
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.999% trace metals basis
form
powder and chunks
impurities
≤20.0 ppm Trace Metal Analysis
density
5.68 g/mL at 25 °C (lit.)
SMILES string
[Ag]
InChI
1S/Ag.HI/h;1H/q+1;/p-1
InChI key
MSFPLIAKTHOCQP-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B J Morgan et al.
Journal of physics. Condensed matter : an Institute of Physics journal, 24(27), 275303-275303 (2012-06-21)
Extreme room temperature conductivity enhancements have been reported for nanocrystalline AgI of up to × 10(4) relative to bulk β-AgI (Guo et al 2005 Adv. Mater. 17 2815-9). These samples were identified as possessing 7H and 9R polytype structures, which can be considered
Olga Alcaraz et al.
The Journal of chemical physics, 134(1), 014505-014505 (2011-01-12)
The structure of molten AgCl, AgI, and their eutectic mixture Ag(Cl(0.43)I(0.57)) is studied by means of molecular dynamics simulations of polarizable ion model potentials. The corresponding static coherent structure factors reproduce quite well the available neutron scattering data. The qualitative
Guoxiang Wang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(4), 821-824 (2010-08-31)
GeS₂-In₂S₃-KI/AgI chalcohalide glasses were prepared by traditional melt-quenching method and the glass-forming region was determined. The dependence of glass properties on composition as formula of (100 - x)(0.75GeS₂-0.25In₂S₃) - x(In₂S₃-KI/AgI) was investigated. The allowed indirect transition of samples was calculated
Takeshi Matsumoto et al.
Inorganic chemistry, 50(7), 2859-2869 (2011-03-11)
The synthesis and characterization of two coordination polymers, {Cu(I)[Mo(V)(bdt)(3)]·0.5Et(2)O}(n) (1·0.5Et(2)O, bdt: o-benzenedithiolato) and {Ag(I)[Mo(V)(bdt)(3)]}(n) (2), composed of redox-active [Mo(V)(bdt)(3)](-) metalloligand with Cu(I) and Ag(I) ions are reported. The complexation reactions of [Mo(V)(bdt)(3)](-) with Cu(II)(ClO(4))(2) or Ag(I)ClO(4) commonly lead to the
Teslima Daşbaşi et al.
Talanta, 103, 1-7 (2012-12-04)
A simple and reliable on-line separation/preconcentration procedure was developed for the determination of trace levels of Ag(I) by flame atomic absorption spectrometry. Poly[N-(3-methyl-1H-indol-1-yl)-2-methacrylamide-co-2-acrylamido-2-methyl-1-propane sulfonic acid-co-divinylbenzene] was synthesized and characterized as a new chelating resin for the first time. Ag(I) was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service