D2141
6-Diazo-5-oxo-L-norleucine
>95% (TLC), suitable for ligand binding assays
Sinónimos:
(S)-2-Amino-6-diazo-5-oxocaproic acid, DON
About This Item
Productos recomendados
Nombre del producto
6-Diazo-5-oxo-L-norleucine, crystalline
Ensayo
>95% (TLC)
Nivel de calidad
Formulario
crystalline
técnicas
ligand binding assay: suitable
color
light yellow
mp
145 °C
espectro de actividad antibiótica
neoplastics
Modo de acción
enzyme | inhibits
temp. de almacenamiento
−20°C
cadena SMILES
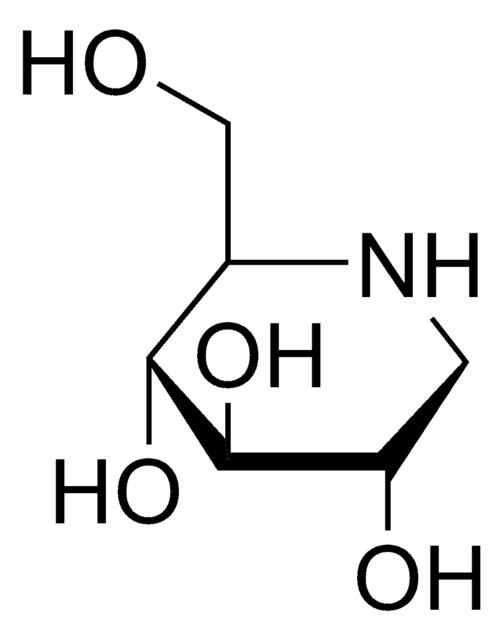
N[C@@H](CCC(=O)C=[N+]=[N-])C(O)=O
InChI
1S/C6H9N3O3/c7-5(6(11)12)2-1-4(10)3-9-8/h3,5H,1-2,7H2,(H,11,12)/t5-/m0/s1
Clave InChI
YCWQAMGASJSUIP-YFKPBYRVSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Sigma article discusses tumor cell metabolic pathways, focusing on aerobic glycolysis and mitochondrial activity.
Sigma article discusses tumor cell metabolic pathways, focusing on aerobic glycolysis and mitochondrial activity.
Sigma article discusses tumor cell metabolic pathways, focusing on aerobic glycolysis and mitochondrial activity.
Sigma article discusses tumor cell metabolic pathways, focusing on aerobic glycolysis and mitochondrial activity.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico