48760
Gentamicin sulfate from Micromonospora purpurea
≥590 μg/mg of gentamicin
Sinónimos:
Gentamicin C1
About This Item
Productos recomendados
origen biológico
Micromonospora purpurea
Nivel de calidad
Formulario
powder
actividad óptica
[α]/D 107.0 to 121.0°, c = 10% in H2O
actividad específica
≥590 μg/mg of gentamicin
mol peso
Mr 694-723
pérdida
≤18.0% loss on drying
color
white to off-white
pH
3.5-5.5 (4% in H2O)
espectro de actividad antibiótica
Gram-negative bacteria
Gram-positive bacteria
mycoplasma
Modo de acción
protein synthesis | interferes
temp. de almacenamiento
2-8°C
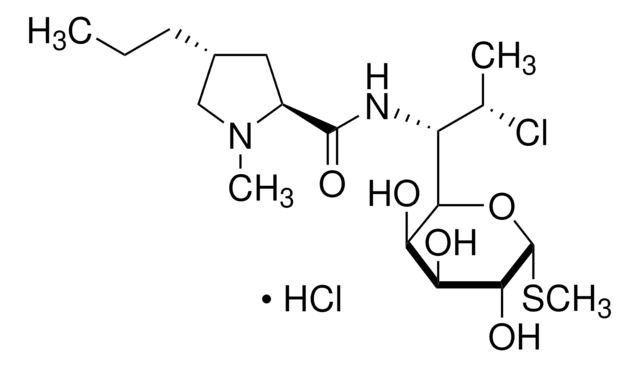
cadena SMILES
OS(O)(=O)=O.CN[C@@H]1[C@@H](O)[C@H](OC[C@]1(C)O)O[C@H]2[C@H](N)C[C@H](N)[C@@H](O[C@H]3O[C@H](CN)CC[C@H]3N)[C@@H]2O.CN[C@@H]4[C@@H](O)[C@H](OC[C@]4(C)O)O[C@H]5[C@H](N)C[C@H](N)[C@@H](O[C@H]6O[C@@H](CC[C@H]6N)[C@@H](C)N)[C@@H]5O.CN[C@H](C)[C@@H]7CC[C@@H](N)[C@H](O7)O[C@@H]8[C@@H](N)C[C@@H](N)[C@H](O[C@H]9OC[C@](C)(O)[C@H](NC)[C@H]9O)[C@H]8O
InChI
1S/C21H43N5O7.C20H41N5O7.C19H39N5O7.H2O4S/c1-9(25-3)13-6-5-10(22)19(31-13)32-16-11(23)7-12(24)17(14(16)27)33-20-15(28)18(26-4)21(2,29)8-30-20;1-8(21)12-5-4-9(22)18(30-12)31-15-10(23)6-11(24)16(13(15)26)32-19-14(27)17(25-3)20(2,28)7-29-19;1-19(27)7-28-18(13(26)16(19)24-2)31-15-11(23)5-10(22)14(12(15)25)30-17-9(21)4-3-8(6-20)29-17;1-5(2,3)4/h9-20,25-29H,5-8,22-24H2,1-4H3;8-19,25-28H,4-7,21-24H2,1-3H3;8-18,24-27H,3-7,20-23H2,1-2H3;(H2,1,2,3,4)/t9-,10-,11+,12-,13+,14+,15-,16-,17+,18-,19-,20-,21+;8-,9-,10+,11-,12+,13+,14-,15-,16+,17-,18-,19-,20+;8-,9+,10-,11+,12-,13+,14+,15-,16+,17+,18+,19-;/m110./s1
Clave InChI
RDEIXVOBVLKYNT-HDZPSJEVSA-N
Categorías relacionadas
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Antimicrobial spectrum: Gram-negative and Gram-positive bacteria, and mycoplasma.
Envase
Definición de unidad
Nota de preparación
Otras notas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Skin Sens. 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico