00140585
Isoquercitrin
primary reference standard
Sinónimos:
Quercetin 3-β-D-glucoside, 3,3′,4′,5,7-Pentahydroxyflavone 3-β-glucoside, Isoquercitrin
About This Item
Productos recomendados
grado
primary reference standard
caducidad
limited shelf life, expiry date on the label
fabricante / nombre comercial
HWI
técnicas
HPLC: suitable
gas chromatography (GC): suitable
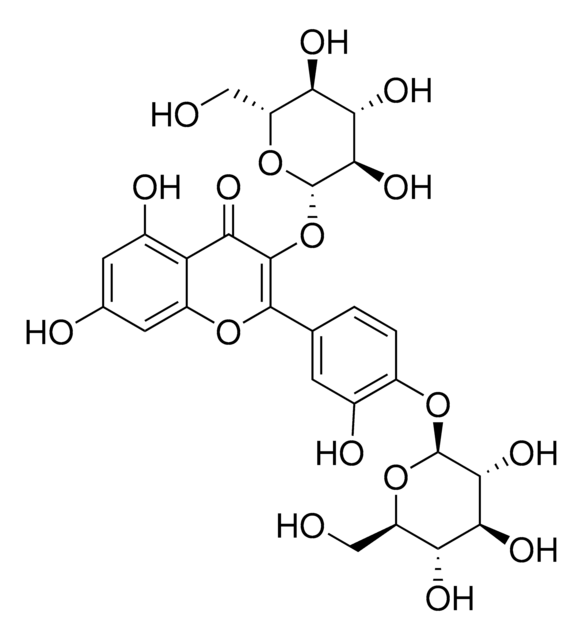
cadena SMILES
OC[C@H]1O[C@@H](OC2=C(Oc3cc(O)cc(O)c3C2=O)c4ccc(O)c(O)c4)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15-,17+,18-,21+/m1/s1
Clave InChI
OVSQVDMCBVZWGM-QSOFNFLRSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Exact content measured by quantitative NMR can be found on the certificate.
Aplicación
- Development of a synergistic cloud point extraction (SCPE) method for the extraction of isoquercitrin in rat plasma samples and its subsequent analysis by high-performance liquid chromatography (HPLC)
- Electrochemical analysis of isoquercitrin and epigallocatechin gallate by cyclic voltammetry using carbon nanotubes based electrode
- Simultaneous determination of rutin and isoquercitrin by differential pulse voltammetry (DPV) using glassy carbon electrode (GCE) modified with deposition of thio-β-cyclodextrin functionalized graphene/palladium nanoparticles (SH-β-CD-Gr/PdNPs) for pharmaceutical and medical analysis
- Multi-residue analysis of isoquercitrin, campesterol, emodin 8-O-β-D-glucopyranoside, and quercetin in dried stem and flower extracts of Reynoutria sachalinensis by high-performance liquid chromatography coupled with diode-array detection
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
HPLC Analysis of Polyphenols in Nero d'Avola Red Wine on Discovery® HS C18 (UV 280 nm)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico