P38706
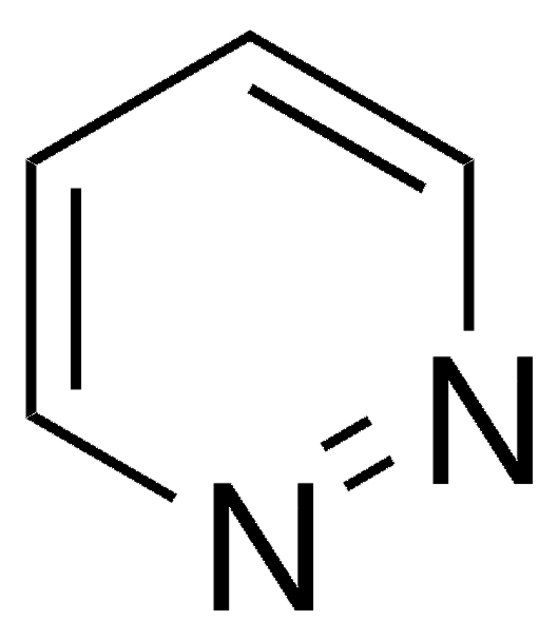
Phthalazine
98%
Sinónimos:
β-Phenodiazine, 2,3-Benzodiazine, 2,3-Diazanaphthalene, Benzo[d]pyridazine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H6N2
Número de CAS:
Peso molecular:
130.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
crystals
bp
189 °C/29 mmHg (lit.)
mp
89-92 °C (lit.)
storage temp.
2-8°C
SMILES string
c1ccc2cnncc2c1
InChI
1S/C8H6N2/c1-2-4-8-6-10-9-5-7(8)3-1/h1-6H
InChI key
LFSXCDWNBUNEEM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 3 - Muta. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Manuel Sánchez-Moreno et al.
The Journal of antimicrobial chemotherapy, 67(2), 387-397 (2011-12-01)
To evaluate the in vitro leishmanicidal activity of imidazole-based (1-4) and pyrazole-based (5-6) benzo[g]phthalazine derivatives against Leishmania infantum and Leishmania braziliensis. The in vitro activity of compounds 1-6 was assayed on extracellular promastigote and axenic amastigote forms, and on intracellular
Fadi M Awadallah et al.
European journal of medicinal chemistry, 52, 14-21 (2012-03-24)
New phthalazine-based vasodilators were synthesized through the chloroacylation of the starting compound 1-hydrazinophthalazine 4 to give the two key intermediates 5 and 7. These intermediates were used to alkylate various cyclic amines to furnish the final compounds 6a-h and 8a-h.
Michał Achmatowicz et al.
The Journal of organic chemistry, 74(2), 795-809 (2008-12-18)
p38 MAP kinase inhibitors have attracted considerable interest as potential agents for the treatment of inflammatory diseases. Herein, we describe a concise and efficient synthesis of inhibitor 1 that is based on a phthalazine scaffold. Highlights of our approach include
Mehdi Rashidi et al.
Inorganic chemistry, 49(18), 8435-8443 (2010-08-18)
The reaction of phthalazine with the binuclear organoplatinum complexes [Me(2)Pt(μ-SMe(2))(μ-dppm)PtR(2)], R = Me, Ph, 4-tolyl or R(2) = (CH(2))(4), dppm = bis(diphenylphosphino)methane, gives the corresponding complexes [Me(2)Pt(μ-phthalazine)(μ-dppm)PtR(2)] by displacement of the bridging dimethylsulfide ligand. The structures of [Me(2)Pt(μ-SMe(2))(μ-dppm)PtMe(2)] and [Me(2)Pt(μ-phthalazine)(μ-dppm)PtMe(2)]
Hyungchul Kim et al.
International journal of pharmaceutics, 377(1-2), 105-111 (2009-05-26)
Investigation of the use of solution NMR spectroscopy to determine the effect of organic solvents on chemical shift changes in bases on addition of acids is reported. This information can be useful in the evaluation of solvents and counterion selection
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico