D122602
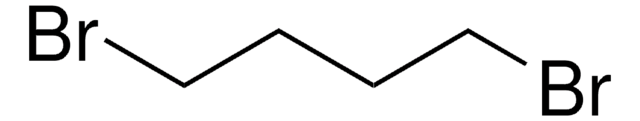
1,4-Diiodobutane
≥99%, contains copper as stabilizer
Sinónimos:
Tetramethylene diiodide
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
I(CH2)4I
Número de CAS:
Peso molecular:
309.92
Beilstein/REAXYS Number:
1098276
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥99%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.621 (lit.)
bp
147-152 °C/26 mmHg (lit.)
mp
6 °C (lit.)
density
2.35 g/mL at 25 °C (lit.)
SMILES string
ICCCCI
InChI
1S/C4H8I2/c5-3-1-2-4-6/h1-4H2
InChI key
ROUYUBHVBIKMQO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Gas chromatographic determination of busulfan in plasma with electron-capture detection.
M Hassan et al.
Journal of chromatography, 277, 374-380 (1983-10-14)
D H Marchand et al.
Drug metabolism and disposition: the biological fate of chemicals, 16(1), 85-92 (1988-01-01)
gamma-Glutamyl-beta-(S-tetrahydrothiophenium)alanyl-glycine, the glutathione-sulfonium conjugate of busulfan and 1,4-diiodobutane, was identified in the bile of rats following intravenous administration of equimolar doses of either compound. The glutathione-sulfonium conjugate was synthesized from 1-bromo-4-chlorobutane and characterized by 1H and 13C NMR and FAB/MS.
D H Marchand et al.
Biochemical and biophysical research communications, 128(1), 360-367 (1985-04-16)
Rat liver glutathione S-transferases catalyzed the conjugation of 1,4-diiodobutane with glutathione in vitro. The reaction followed saturation kinetics and was dependent on the concentration of the enzyme, substrate and glutathione in the incubation media. S-Benzylglutathione inhibited the enzymatic conversion of
Ian C Watson et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(41), 9678-9690 (2019-05-16)
New N-heterocyclic olefins (NHOs) are described with functionalization on the ligand heterocyclic backbone and terminal alkylidene positions. Various PdII -NHO complexes have been formed and their use as pre-catalysts in Buchwald-Hartwig aminations was explored. The most active system for catalytic
Jianghong Cai et al.
Molecules (Basel, Switzerland), 25(20) (2020-10-18)
In comparison with pristine sinomenine and carborane precursors, the calculations of molecular docking with matrix metalloproteinases (MMPs) and methylcarboranyl-n-butyl sinomenine showed improved interactions. Accordingly, methylcarboranyl-n-butyl sinomenine shows a high potential in the treatment of rheumatoid arthritis (RA) in the presence
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico