ALD00158
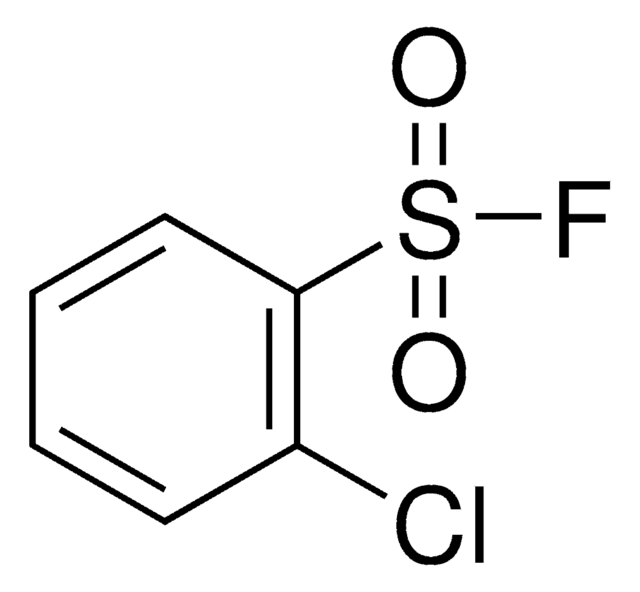
5-Chloro-2-thiophenesulfonyl fluoride
95%
About This Item
Productos recomendados
Quality Level
assay
95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.529
density
1.571 g/mL at 25 °C
SMILES string
ClC1=CC=C(S(F)(=O)=O)S1
InChI
1S/C4H2ClFO2S2/c5-3-1-2-4(9-3)10(6,7)8/h1-2H
InChI key
XZQCGAGVTAESGC-UHFFFAOYSA-N
Categorías relacionadas
Application
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
Contenido relacionado
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico