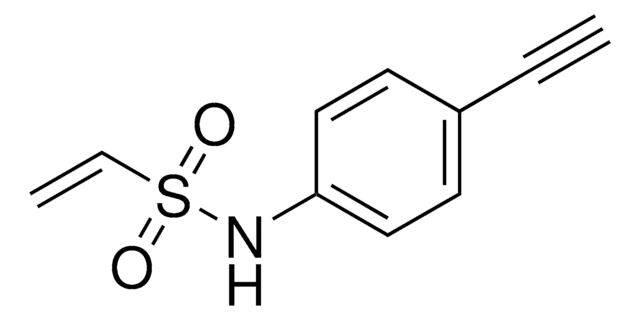
927589
ArVS-alkyne
≥95%
Sinónimos:
N-(Prop-2-yn-1-yl)-4-(vinylsulfonyl)benzamide
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H11NO3S
Peso molecular:
249.29
UNSPSC Code:
12352101
NACRES:
NA.22
Productos recomendados
Application
ArVS-alkyne is a Michael acceptor probe that can be used to label cysteines. A method was developed using cysteine-reactive compounds including this one to allow for unbiased analysis of proteomic data in quantitative applications . The method uses light or heavy labeling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis . Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Profiling the proteome-wide selectivity of diverse electrophiles.
Zanon P RA, et al.
ChemRxiv : the preprint server for chemistry (2021)
Patrick R A Zanon et al.
Angewandte Chemie (International ed. in English), 59(7), 2829-2836 (2019-11-30)
Rapid development of bacterial resistance has led to an urgent need to find new druggable targets for antibiotics. In this context, residue-specific chemoproteomic approaches enable proteome-wide identification of binding sites for covalent inhibitors. Described here are easily synthesized isotopically labeled
Keriann M Backus et al.
Nature, 534(7608), 570-574 (2016-06-17)
Small molecules are powerful tools for investigating protein function and can serve as leads for new therapeutics. Most human proteins, however, lack small-molecule ligands, and entire protein classes are considered 'undruggable'. Fragment-based ligand discovery can identify small-molecule probes for proteins
Eranthie Weerapana et al.
Nature, 468(7325), 790-795 (2010-11-19)
Cysteine is the most intrinsically nucleophilic amino acid in proteins, where its reactivity is tuned to perform diverse biochemical functions. The absence of a consensus sequence that defines functional cysteines in proteins has hindered their discovery and characterization. Here we
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico