912131
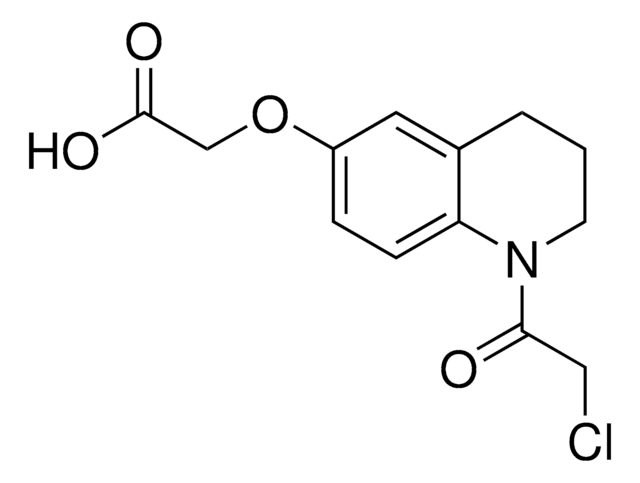
2-Chloro-1-(6-methoxy-1,2,3,4-tetrahydroquinolin-1-yl)ethan-1-one
≥95%
Sinónimos:
1-(Chloroacetyl)-1,2,3,4-tetrahydro-6-quinolinyl methyl ether, 2-Chloro-1-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)ethan-1-one, Electrophilic scout fragment, KB02, Scout fragment for targetable cysteine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H14ClNO2
Número de CAS:
Peso molecular:
239.70
MDL number:
UNSPSC Code:
12352200
NACRES:
NA.22
Productos recomendados
Application
2-Chloro-1-(6-methoxy-1,2,3,4-tetrahydroquinolin-1-yl)ethan-1-one is a cysteine-reactive small-molecule fragment for chemoproteomic and ligandability studies for both traditionally druggable proteins as well as "undruggable," or difficult-to-target, proteins. This fragment electrophile, or "scout" fragment, can be used alone in fragment-based covalent ligand discovery or incorporated into bifunctional tools such as electrophilic PROTAC® molecules for targeted protein degradation as demonstrated by the Cravatt Lab Lab for E3 ligase discovery.
Other Notes
Legal Information
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
related product
Referencia del producto
Descripción
Precios
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Kristine Senkane et al.
Angewandte Chemie (International ed. in English), 58(33), 11385-11389 (2019-06-22)
Reversible covalency, achieved with, for instance, highly electron-deficient olefins, offers a compelling strategy to design chemical probes and drugs that benefit from the sustained target engagement afforded by irreversible compounds, while avoiding permanent protein modification. Reversible covalency has mainly been
Xiaoyu Zhang et al.
Nature chemical biology, 15(7), 737-746 (2019-06-19)
Ligand-dependent protein degradation has emerged as a compelling strategy to pharmacologically control the protein content of cells. So far, however, only a limited number of E3 ligases have been found to support this process. Here, we use a chemical proteomic
Keriann M Backus et al.
Nature, 534(7608), 570-574 (2016-06-17)
Small molecules are powerful tools for investigating protein function and can serve as leads for new therapeutics. Most human proteins, however, lack small-molecule ligands, and entire protein classes are considered 'undruggable'. Fragment-based ligand discovery can identify small-molecule probes for proteins
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico