676403
(R,R)-(–)-2,3-Bis(tert-butylmethylphosphino)quinoxaline
≥95%
Sinónimos:
(R) QuinoxP®
About This Item
Productos recomendados
Quality Level
assay
≥95%
form
solid
mp
100-104 °C
functional group
phosphine
SMILES string
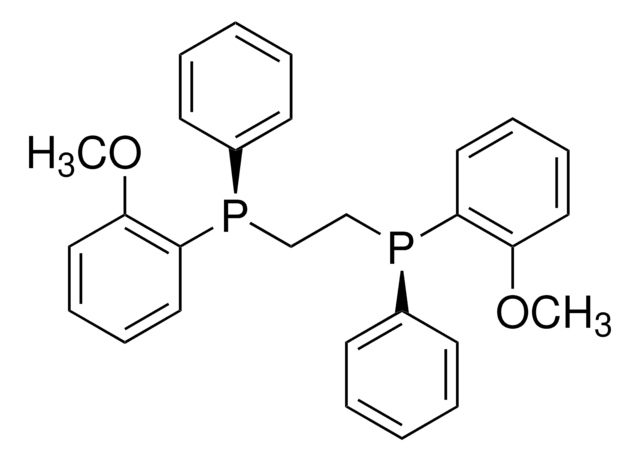
CP(c1nc2ccccc2nc1P(C)C(C)(C)C)C(C)(C)C
InChI
1S/C18H28N2P2/c1-17(2,3)21(7)15-16(22(8)18(4,5)6)20-14-12-10-9-11-13(14)19-15/h9-12H,1-8H3/t21-,22-/m0/s1
InChI key
DRZBLHZZDMCPGX-VXKWHMMOSA-N
Categorías relacionadas
General description
Application
Features and Benefits
- It is not oxidized nor epimerized at ambient conditions in air
- Enantioselectivities are outstanding for various reaction paradigms
- Hydrogenations proceed under mild reaction conditions
- Low catalyst loadings yield high TONs
Citation
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
QuinoxP*: Air-Stable and Highly Efficient and Productive Chiral Ligands
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)
![(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)