654213
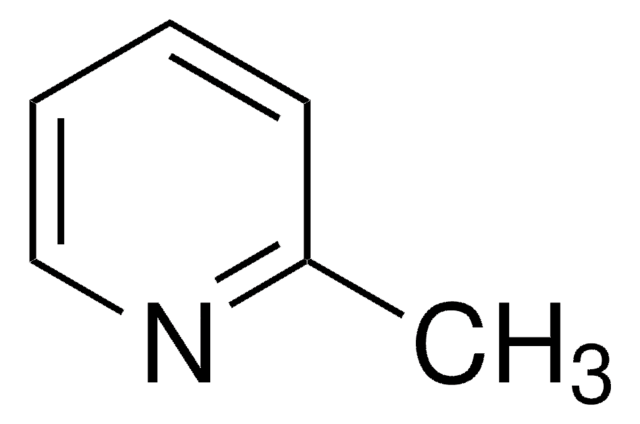
2-Methylpyridine borane complex
95%
Sinónimos:
2-Picoline borane complex
About This Item
Productos recomendados
Quality Level
assay
95%
form
solid
reaction suitability
reagent type: reductant
storage temp.
2-8°C
SMILES string
CC1=NC=CC=C1.B
InChI
1S/C6H7N.B/c1-6-4-2-3-5-7-6;/h2-5H,1H3;
InChI key
QHXLIQMGIGEHJP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Synthesis of alkoxyamine derivatives, via reduction of oxime ethers.
- Reductive amination reactions of C1-C10 aldehyde 2,4-dinitrophenylhydrazones.
- Synthesis of various trifluoromethylated amino compounds.
- An alternative reagent for reductive aminations.
Reactant for:
Reductive amination
N-Benzyl-protection of amino acid derivatives by reductive alkylation
Reductive alkoxyamination
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3 - Water-react 2
target_organs
Respiratory system
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
212.0 °F - closed cup
flash_point_c
100 °C - closed cup
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
2-picoline-borane (pic-BH3) is an excellent alternative reagent for reductive aminations.
Learn about the applications of chiral oxazaborolidinium ions (COBIs) as Lewis acid catalysts in different asymmetric reactions such as cyclopropanation, epoxidation, and radical reactions along with details of their catalytic action.
Learn about the applications of chiral oxazaborolidinium ions (COBIs) as Lewis acid catalysts in different asymmetric reactions such as cyclopropanation, epoxidation, and radical reactions along with details of their catalytic action.
Learn about the applications of chiral oxazaborolidinium ions (COBIs) as Lewis acid catalysts in different asymmetric reactions such as cyclopropanation, epoxidation, and radical reactions along with details of their catalytic action.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico