651664
Tetrabutylammonium azide
Sinónimos:
N,N,N-Tributyl-1-butanaminium azide, Tetrabutylammonium nitride
About This Item
Productos recomendados
form
solid
reaction suitability
reaction type: click chemistry
mp
84-88 °C (lit.)
functional group
amine
azide
storage temp.
2-8°C
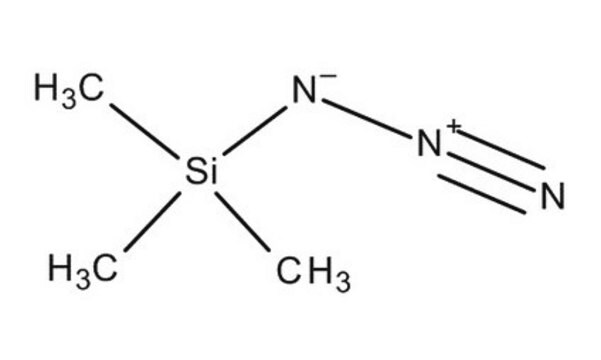
SMILES string
[N-]=[N+]=[N-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.N3/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-3-2/h5-16H2,1-4H3;/q+1;-1
InChI key
GMRIOAVKKGNMMV-UHFFFAOYSA-N
Categorías relacionadas
Application
- Heteroarylannulated bicyclic morpholines
- Cyanimide-based inhibitors of cathepsin C
- Trimethylene carbonate
Reagent for:
- Aerobic oxidative transformation of primary azides to nitriles
- Substitution reactions at tetracoordinate boron
Catalyst for syslic carbonate formation
- Heteroarylannulated bicyclic morpholines
- Cyanimide-based inhibitors of cathepsin C
- Trimethylene carbonate
- Aerobic oxidative transformation of primary azides to nitriles
- Substitution reactions at tetracoordinate boron
It is also used as a catalyst for cyclic carbonate formation.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
supp_hazards
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico