638439
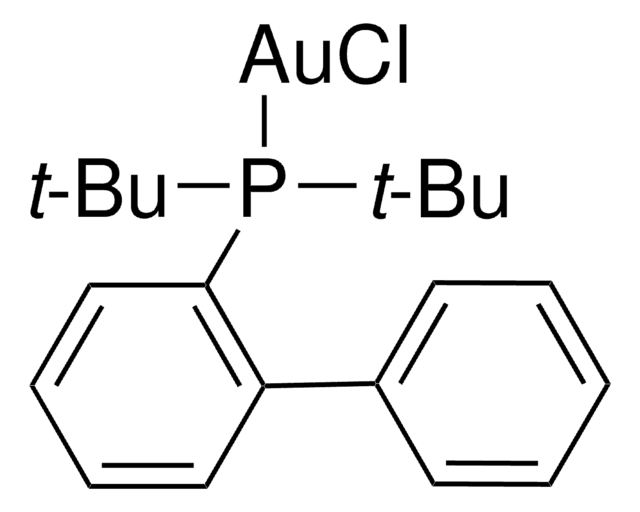
(2-Biphenyl)di-tert-butylphosphine
97%
Sinónimos:
(2-Biphenylyl)di-tert-butylphosphine
About This Item
Productos recomendados
Quality Level
assay
97%
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
86-88 °C (lit.)
functional group
phosphine
SMILES string
CC(C)(C)P(c1ccccc1-c2ccccc2)C(C)(C)C
InChI
1S/C20H27P/c1-19(2,3)21(20(4,5)6)18-15-11-10-14-17(18)16-12-8-7-9-13-16/h7-15H,1-6H3
InChI key
CNXMDTWQWLGCPE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Learn more about Buchwald Phosphine Ligands
Application
- Hydrophenoxylation of unactivated internal alkynes.
- Microwave-mediated Suzuki-Miyaura cross-coupling of benzylic bromides.
- Pharmaceutical synthesis of novel imidazo[1,2-a]pyridines, having potent activity against the herpes virus.
- Barluenga′s coupling of vinyl bromides with hydrazines.
- Pd-catalyzed 2,3-diarylation of α,α-disubstituted-3-thiophenemethanols, via cleavage of C-H and C-C bonds.
Catalyst for:
- Decarboxylative cross-coupling of dialkoxybenzoic acids with diaryl disulfides or diaryl diselenides
- Stereoselective preparation of imidazolidinones via intramolecular hydroamination of N-allylic-N-arylureas
- Regioselective arylation of olefins with aryl chlorides
- Cross-coupling reaction for the synthesis of polyunsaturated macrolactones
- Regioselective O-alkylation reactions
- Sonogashira-type cross coupling
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Buchwald and coworkers develop versatile phosphine ligands for Pd-catalyzed C–N bond formation; enhancing synthetic reactions for 20 years.
Buchwald and coworkers develop versatile phosphine ligands for Pd-catalyzed C–N bond formation; enhancing synthetic reactions for 20 years.
Buchwald and coworkers develop versatile phosphine ligands for Pd-catalyzed C–N bond formation; enhancing synthetic reactions for 20 years.
Buchwald and coworkers develop versatile phosphine ligands for Pd-catalyzed C–N bond formation; enhancing synthetic reactions for 20 years.
Contenido relacionado
La plataforma Catalexis mejora la catálisis al optimizar digitalmente la selección de catalizadores para identificar los ligandos de fosfina más eficaces para las reacciones de acoplamiento cruzado.
La plataforma Catalexis mejora la catálisis al optimizar digitalmente la selección de catalizadores para identificar los ligandos de fosfina más eficaces para las reacciones de acoplamiento cruzado.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico