576638
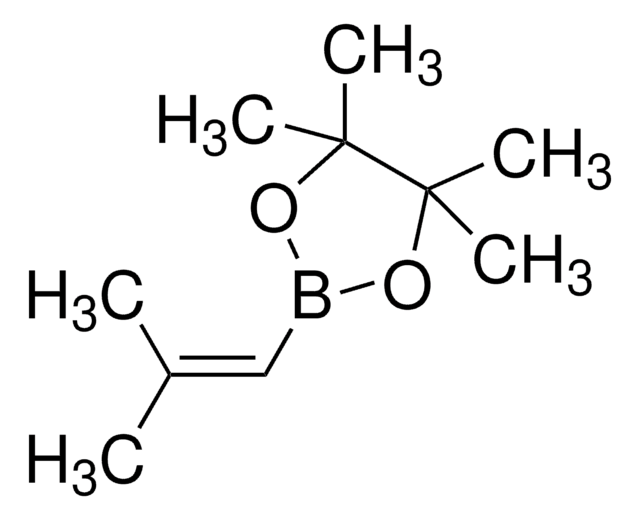
trans-1-Propen-1-ylboronic acid
≥95.0%
Sinónimos:
(E)-1-Propen-1-ylboronic acid, (E)-Prop-1-enylboronic acid, trans-1-Propeneboronic acid, trans-1-Propenylboronic acid, trans-Propenylboronic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3CH=CHB(OH)2
Número de CAS:
Peso molecular:
85.90
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥95.0%
impurities
~10 wt. % cis-isomer
mp
123-127 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(C)=C(\[H])B(O)O
InChI
1S/C3H7BO2/c1-2-3-4(5)6/h2-3,5-6H,1H3/b3-2+
InChI key
CBMCZKMIOZYAHS-NSCUHMNNSA-N
Application
Reactant for:
Reactant for preparation of:
- Palladium-phosphine-catalyzed Suzuki-Miyaura coupling reactions
- Cu(II)-mediated Ullmann-type coupling
Reactant for preparation of:
- Alkynylphenoxyacetic acids as DP2 receptor antagonists for treatment of allergic inflammatory diseases
- Tetrahydrobenzothiophenes as conformationally restricted enol-mimic inhibitors of type II dehydroquinase via Paal-Knorr synthesis involving Suzuki coupling
- Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-mediated cyclization
- Stereospecific dienes via nickel-catalyzed three-component reductive coupling with alkynes and enones
Reactant for
Reactant for preparation of
- Palladium-phosphine-catalyzed Suzuki-Miyaura coupling reactions
- Cu(II)-mediated Ullmann-type coupling
- Palladium-catalyzed Sonogashira cross-coupling
Reactant for preparation of
- Alkynylphenoxyacetic acids as DP2 receptor antagonists for treatment of allergic inflammatory diseases
- Tetrahydrobenzothiophenes as conformationally restricted enol-mimic inhibitors of type II dehydroquinase via Paal-Knorr synthesis involving Suzuki coupling
- Highly substituted benzannulated cyclooctanol derivatives by samarium diiodide-mediated cyclization
- Stereospecific dienes via nickel-catalyzed three-component reductive coupling with alkynes and enones
Other Notes
Contains varying amounts of anhydride
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chun-Ming Yang et al.
Organic letters, 12(16), 3610-3613 (2010-08-14)
A highly regio- and stereoselective nickel-catalyzed three-component coupling of alkynes with enones and alkenyl boronic acids to afford highly substituted 1,3-dienes is described. The reaction can also be extended to cyclization of enynes with coupling to alkenyl boronic acids. A
Total synthesis of rodgersinol: a survey of the Cu(II)-mediated coupling of ortho-substituted phenols
Jung, J.-W.; et al.
Tetrahedron, 66, 6826-6831 (2010)
A new strategy for the synthesis of substituted dihydropyrones and tetrahydropyrones via palladium-catalyzed coupling of thioesters
Fuwa, H.; et al.
Tetrahedron, 67, 4995-5010 (2011)
Tetrahydrobenzothiophene derivatives: conformationally restricted inhibitors of type II dehydroquinase.
Sonia Paz et al.
ChemMedChem, 6(2), 266-272 (2011-01-29)
Ming-Bo Zhou et al.
The Journal of organic chemistry, 75(16), 5635-5642 (2010-08-14)
Palladium-catalyzed cross-coupling reaction of terminal alkynes with arylboronic acids has been described. In the presence of Pd(OAc)(2) and Ag(2)O, a variety of terminal alkynes, including electron-poor terminal alkynes, smoothly underwent the reaction with numerous boronic acids to afford the corresponding
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico