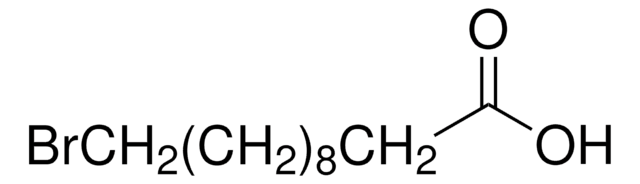447463
Methyl 11-bromoundecanoate
95%
Sinónimos:
11-Bromoundecanoic acid methyl ester
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Br(CH2)10CO2CH3
Número de CAS:
Peso molecular:
279.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
95%
refractive index
n20/D 1.465 (lit.)
bp
115 °C/0.04 mmHg (lit.)
density
1.157 g/mL at 25 °C (lit.)
SMILES string
COC(=O)CCCCCCCCCCBr
InChI
1S/C12H23BrO2/c1-15-12(14)10-8-6-4-2-3-5-7-9-11-13/h2-11H2,1H3
InChI key
HFNPVFKUZYCDIB-UHFFFAOYSA-N
Categorías relacionadas
Application
Methyl 11-bromoundecanoate can be used as a reactant to synthesize:
- Methyl 11-(2,5-dibromophenoxy)undecanoate, which is employed as a precursor to prepare acetylenic cyclophanes.
- Methyl 11-[(1-phenyl-1H-tetrazol-5-yl)thio]undecanoate, a key intermediate applicable in the synthesis of emmyguyacins side chain.
- Betain derivatives of 11-bromoundecanoic acid, as potential microbial agents.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Collins et al.
Organic letters, 2(20), 3189-3192 (2000-09-29)
The synthesis of a series of novel acetylenic cyclophanes is described. X-ray crystallographic analysis of the core structure revealed a twisted conformation with helical chirality. Preliminary results suggest that these cyclophanes, with appropriate functionality, have the potential to act as
A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus.
Hassarajani SA and Mamdapur VR.
Molecules (Basel), 3(2), 41-43 (1998)
Synthesis, characterization, antimicrobial and anti-biofilm activity of a new class of 11-bromoundecanoic acid-based betaines
Yasa SR, et al.
Medicinal Chemistry Research, 26(10), 2592-2601 (2017)
Makoto Hashimoto et al.
Bioorganic & medicinal chemistry letters, 12(1), 89-91 (2001-12-12)
A versatile synthesis of diazirine-based photoreactive fatty acid analogues is reported. The key step is phenoxy alkylation of diazirine with halo alkyl acid esters. The conditions described will be acceptable for the synthesis of various alkyl-length derivatives. The fatty acid
Santanu Jana et al.
Organic letters, 20(21), 6938-6942 (2018-10-24)
Fungal glycolipids emmyguyacins A and B inhibit the pH-dependent conformational change of hemaglutinin A during replication of the Influenza virus. Herein, we report the first total synthesis and structure confirmation of emmyguyacins A and B. Our efficient route, which involves
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico