445134
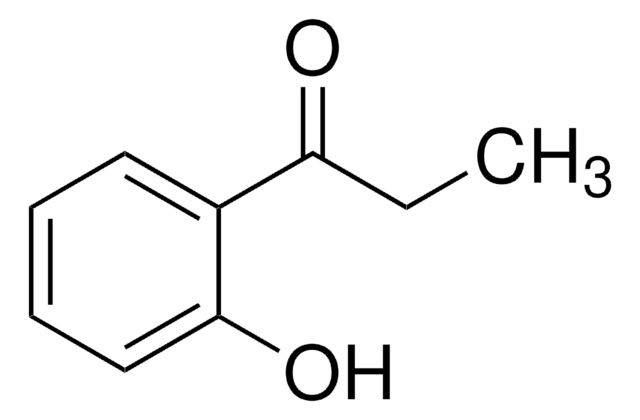
2-Hydroxyacetophenone
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5COCH2OH
Número de CAS:
Peso molecular:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
mp
86-89 °C (lit.)
functional group
hydroxyl
ketone
phenyl
SMILES string
OCC(=O)c1ccccc1
InChI
1S/C8H8O2/c9-6-8(10)7-4-2-1-3-5-7/h1-5,9H,6H2
InChI key
ZWVHTXAYIKBMEE-UHFFFAOYSA-N
Application
2-Hydroxyacetophenone can be used as a starting material for the synthesis of:
- Enantioselective 1R-phenyl-1,2-ethanediol in the presence of a rhodium(III) catalyst by asymmetric transfer hydrogenation.
- Copper(II) complexes of 2-hydroxyacetophenone N-substituted thiosemicarbazones.
- Chromium, molybdenum, and ruthenium complexes of 2-hydroxyacetophenone Schiff bases.
- 2-Hydroxyacetophenone-aroyl hydrazone derivatives for inhibition of copper corrosion in nitric acid.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synthetic studies on optically active Schiff-base ligands derived from condensation of 2-hydroxyacetophenone and chiral diamines.
Gao WT and Zheng Z.
Molecules (Basel), 7(7), 511-516 (2002)
2-Hydroxyacetophenone-aroyl hydrazone derivatives as corrosion inhibitors for copper dissolution in nitric acid solution
AS Fouda, et al.
Bulletin of the Korean Chemical Society,, 21(11), 1085-1089 (2000)
Solvent-free microwave-assisted Beckmann rearrangement of benzaldehyde and 2-hydroxyacetophenone oximes.
Loupy A and Regnier S.
Tetrahedron Letters, 40(34), 6221-6224 (1999)
Mateusz Łużny et al.
Molecules (Basel, Switzerland), 24(17) (2019-09-05)
Biotransformations were performed on eight selected yeast strains, all of which were able to selectively hydrogenate the chalcone derivatives 3-(2"-furyl)- (1) and 3-(2"-thienyl)-1-(2'-hydroxyphenyl)-prop-2-en-1-one (3) into 3-(2"-furyl)- (2) and 3-(2"-thienyl)-1-(2'-hydroxyphenyl)-propan-1-one (4) respectively. The highest efficiency of hydrogenation of the double bond
A stereochemically well-defined rhodium (III) catalyst for asymmetric transfer hydrogenation of ketones
Matharu DS, et al.
Organic Letters, 7(24), 5489-5491 (2005)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico