424757
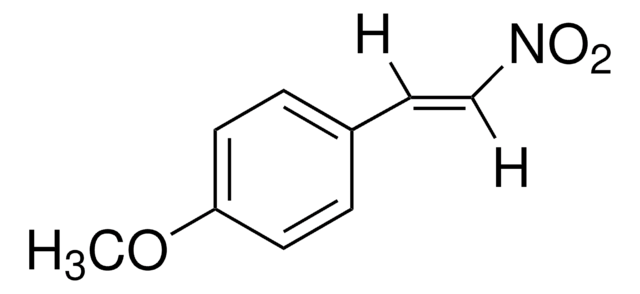
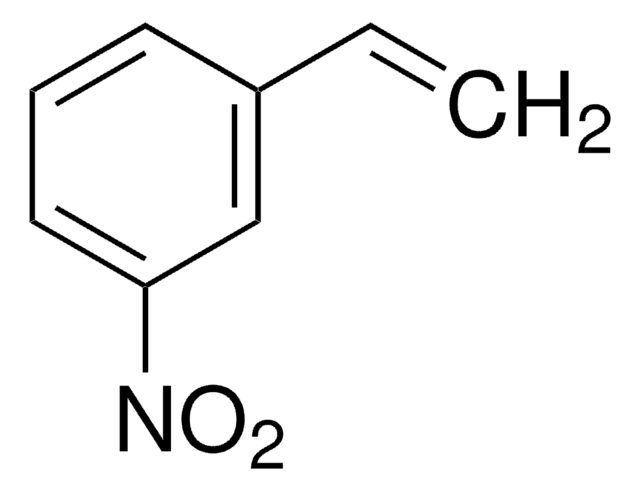
trans-4-Methyl-β-nitrostyrene
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3C6H4CH=CHNO2
Número de CAS:
Peso molecular:
163.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
solid
mp
102-104 °C (lit.)
functional group
amine
nitro
storage temp.
2-8°C
SMILES string
[H]\C(=C(\[H])[N+]([O-])=O)c1ccc(C)cc1
InChI
1S/C9H9NO2/c1-8-2-4-9(5-3-8)6-7-10(11)12/h2-7H,1H3/b7-6+
Inchi Key
JSPNBERPFLONRX-VOTSOKGWSA-N
General description
trans-4-Methyl-β-nitrostyrene ((E)-1-methyl-4-(2-nitrovinyl)benzene) is a nitrolefin. Its asymmetric Michael addition with benzaldehyde in the presence of silylated pyrrolidine catalyst has been reported. Its hydrogenation in the presence of Pd(II) complexes of (Z)-2-((quinolin-3-ylimino)methyl)phenol as catalyst has been studied.
Application
trans-4-Methyl-β-nitrostyrene may be used as a reagent in the synthesis of N-benzylpyrrolomorphinans and 4-oxo-2-aryl-4H-chromene-3-carboxylate derivatives.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Pd (II) complexes based on quinoline derivative: Structural characterization and their role as a catalyst for hydrogenation of (E)-1-methyl-4-(2-nitrovinyl) benzene.
Azam M, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 123, 1-6 (2014)
Silylated pyrrolidines as catalysts for asymmetric Michael additions of aldehydes to nitroolefins.
Ralph Husmann et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(42), 12549-12552 (2010-09-30)
Sanjay K Srivastava et al.
Journal of medicinal chemistry, 45(2), 537-540 (2002-01-11)
A new method for the preparation of N-benzylpyrrolomorphinans has been developed. Thus Michael reaction of the benzylimines of oxycodones and oxymorphones with nitrostyrenes gave a series of 4'-aryl-N-benzylpyrrolomorphinans. These were selective delta antagonists of much higher in vitro potency (with
Manoj R Zanwar et al.
The Journal of organic chemistry, 77(15), 6495-6504 (2012-07-20)
The unusual alcohol mediated formation of 4-oxo-2-aryl-4H-chromene-3-carboxylate (flavone-3-carboxylate) derivatives from 4-hydroxycoumarins and β-nitroalkenes in an alcoholic medium is described. The transformation occurs via the in situ formation of a Michael adduct, followed by the alkoxide ion mediated rearrangement of the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico